Sonesta 6210 User manual

1
User Manual
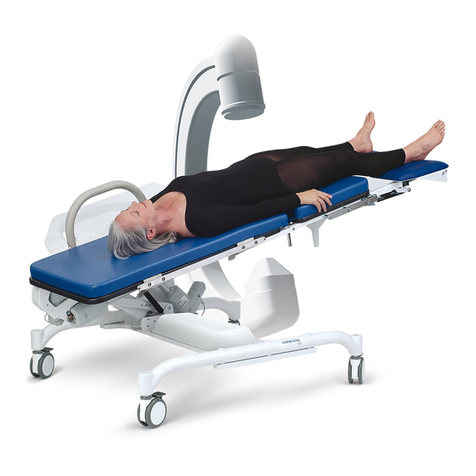
Video Fluoroscopy Table
6210
Sonesta manufactures and sells tables and chairs for urology,
gynecology, urodynamic diagnostics and upper GI procedures

2
Copyright © 2017 Sonesta Medical AB. All rights reserved.
The contents of this manual are the property of SONESTA MEDICAL AB. Any reproduction in whole or in part is strictly
prohibited.
At the time of printing, this manual correctly described the device and its functions. However, as modifications may have
been carried out since the production of this manual, the system package may contain one or more amendments to the
manual. This manual including any such amendments must be thoroughly read, before servicing/using the device.
SONESTA MEDICAL AB is only responsible for the reliability and performance of the device if the following are strictly
observed:
• Authorized personnel (see back of manual for authorized service centers) carry out all repairs and modifications.
• Only Sonesta spare parts are being used.
• The device must be used in accordance with the intended use and standards put forth in the Safety Information section.
If the points above are not strictly observed, the warranty will be considered invalid.
This manual contains different icons and typefaces designed to improve readability and increase understanding of its
content. Adhere to the following recommendations and the ones in the User Manual for safe operation of the device and
service: See below and in the user manual.
NOTE: Sets apart special information or important instruction clarification.
WARNING: The symbol identifies situations or actions that may affect patient or user safety. Disregarding a
warning could result in patient or user injury.
CAUTION: The symbol points out special procedures or precautions that personnel must follow to avoid
equipment damage.
CAUGHT WARNING: The symbol highlights a risk of physical danger to the user or operator (ensure that
hands, feet and equipment are clear of the frame assemblies when changing positions of the chair, always
engage the brakes, verify the stability after brakes are engaged, except during transport).
LIFTING WARNING: The symbol highlights a risk of physical danger to the user or operator when lifting
parts or chair. Use proper lifting methods.

3
Contents........................................................................................................................................................3
Safety Information........................................................................................................................................4
Symbols.........................................................................................................................................................5
Video Fluoroscopy Table 6210.....................................................................................................................6
Controls.........................................................................................................................................................7
Emergency Stop..............................................................................................................................................7
Battery Back Up System .................................................................................................................................7
Pelvic Rotation.................................................................................................................................................8
Standing Position ............................................................................................................................................8
Accessories ..................................................................................................................................................9
Maintenance .................................................................................................................................................11
Cleaning ........................................................................................................................................................11
Mattres Cleaning Instructions.....................................................................................................................12
Technical Data ..............................................................................................................................................13
Measures and Weights.................................................................................................................................13
Imaging Area.................................................................................................................................................14
Classification ................................................................................................................................................14
Technical Lifetime.........................................................................................................................................14
Notes .............................................................................................................................................................15
Service Centers ............................................................................................................................................16
Contents

4
Safety Information
Limitations and Warnings
The product is tested for a patient weight of maximum 250 kg.
- 250 kg
Ensure that the patient does not sit on the footrests, when folded up. The table may become
unstable, causing injury if maximum weight of 50 kg is exceeded.
- 50 kg
Ensure that the patient is never left unattended.
A patient should never enter the chair by using the footrests to stand on while the 6210 is in a
chair position or in a vertical position, unless the footrests are flushed with the floor.
This product is CE marked in conformity with the requirements of the Medical Device Directive 93/42/EEC and the RoHS
Directive 2002/95/ECRoHS.
Adhere to the following recommendations for safe operation of the device:
• Do not attempt to open the control box or actuators.
• Do not immerse the control unit device in water or any other liquid (see the Cleaning section for specific details).
• The power connector also serves as the main power switch i.e. remove the power connector to power off.
• Unplug the power connector from its power source before cleaning or servicing. Failure to do so could result in equip-
ment damage.
• Ensure that the power cord does not become pinched between mechanisms during normal operation of the chair.
Failure to do so can result in equipment damage.
• Ensure that the cables are undammaged before use.
• Do not connect the power connector or any control unit connector to anything other than the appropriate input on the
device.
• The chair is only to be used with medical equipment complying with EN 60601-1. EN 60601-1-2.
• The device is not intended for use with flammable anesthetic gases. A possible explosion hazard exists and personal
injury or equipment damage could occur.
• Ensure that hands, feet and equipment are clear of the frame assemblies when changing positions of the chair.
• Always engage the brakes, except during transport, verify the stability after brakes are engaged.
• Remove drainage receptor and holder/funnel or other option fitted beneath the seat cushion before setting the chair
in standing position.
• A patient should never enter the chair by using the footrests to stand on. This may cause tipping of the chair, and can
cause patient injury.
• The chair is not intended to be used during invasive surgical procedures.
• Use only Sonesta spare parts.
• The device is equipped with a emergency battery back up system built into the control box, the battery is charged when
the AC-powercord is connected to the power source. Always keep the power cord connected to the power source
when in use.
• Make sure the table is in the highest position before setting the table in standing position.
• During patient transport the table must be set to its lowest position (lift motor) so the table does not over balance, this
to avoid personal injuries, equipment damage or property damage.

5
Symbols
Explanations of the symbols found on the body of the device:
2 min per 18 min
Attention, see accompanying documents
Type B, equipment providing a particular degree of protection against electric shock
The device complies with the EC directive 93/42/EEC on medical devices
Degree of protection, EN 60529 (ingress of water and foreign objects)
Maximum safe workload icon, maximum safe workload 250 kg
Duty cycle icon, duty cycle 2 min per 18 min
The UL certification mark
Explanations of the symbols found in the service manual:
Moves up
Moves down
Secure
Release
Push
Insertion direction (part)
Adjustable in shown directions
Adjustable in shown directions
Adjustable in shown directions
Adjustable in shown directions
Potential equalization connection point
IPX6
-250 kg
Battery backup capacity: 10 min with maximum weight
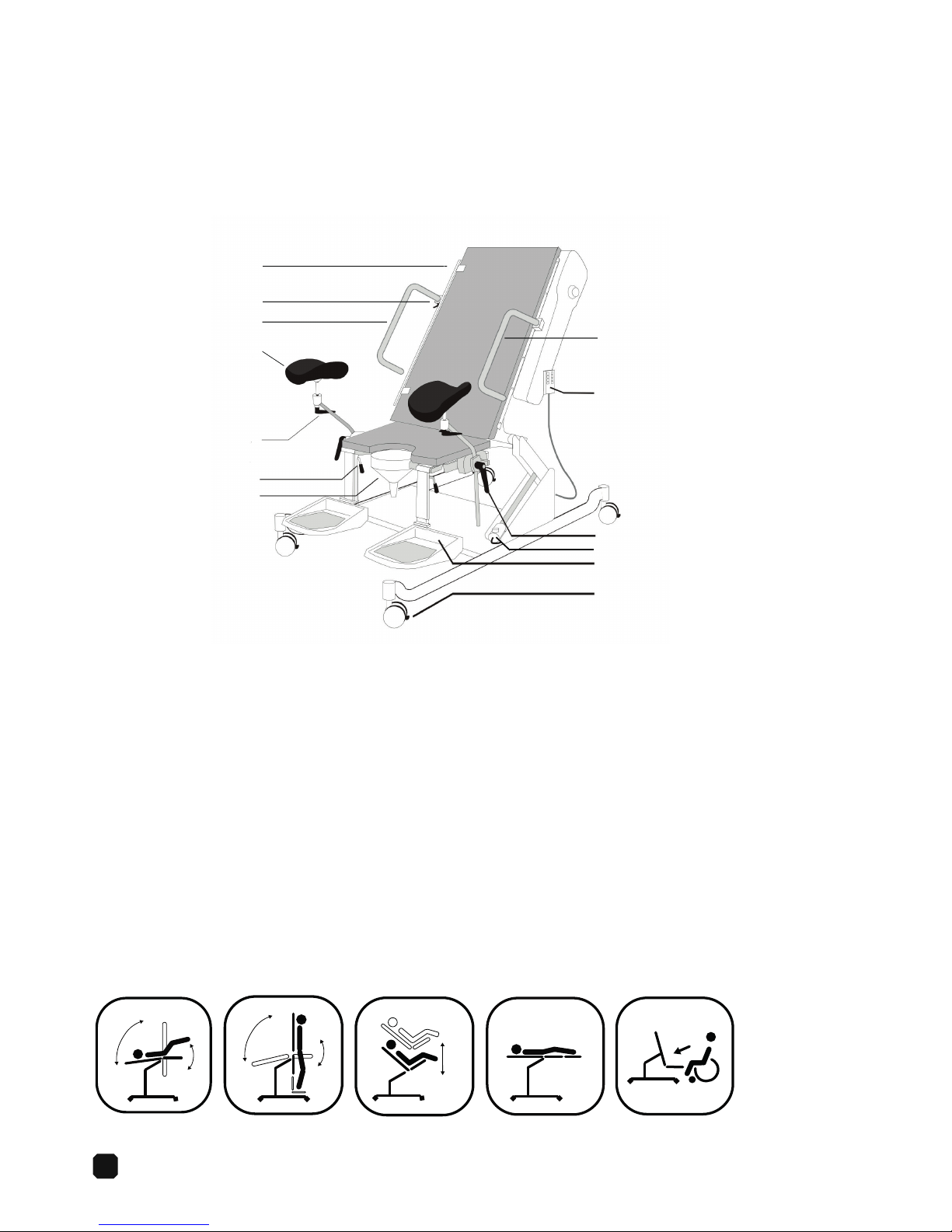
6
1
2
3
4
5
6
7
8
9
10
11
12
13
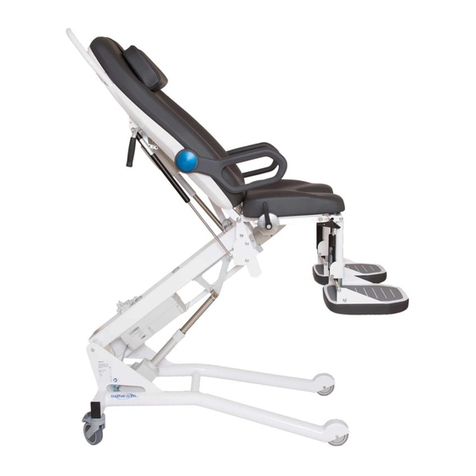
Video Fluoroscopy Table 6210
Intended Use
The 6210 is intended to position the patient in a desired position during urodynamics or
video fluoroscopy examinations and procedures.
Operation range
-15°—+90°
0°90°
-15°—+90°
0°90°
51—137 cm
Description
1. Detachable side rail
2. Detachable arm rest holder
3. Detachable right arm rest
4. Leg rest
5. Leg rest adjustment screw, release screw to adjust angle of the leg rest and then secure.
6. Foot rest adjustment handle, use handle to set position.
7. Funnel, holder and bracket
8. Left arm rest
9. Hand control
10. Screw for adjusting the leg rest and the leg rest holder. Release the screw to adjust the height of the leg rest or the leg
rest holder.
11. Emergency stop knob
12. Fold up foot rests
13. Wheels, with locking mechanism
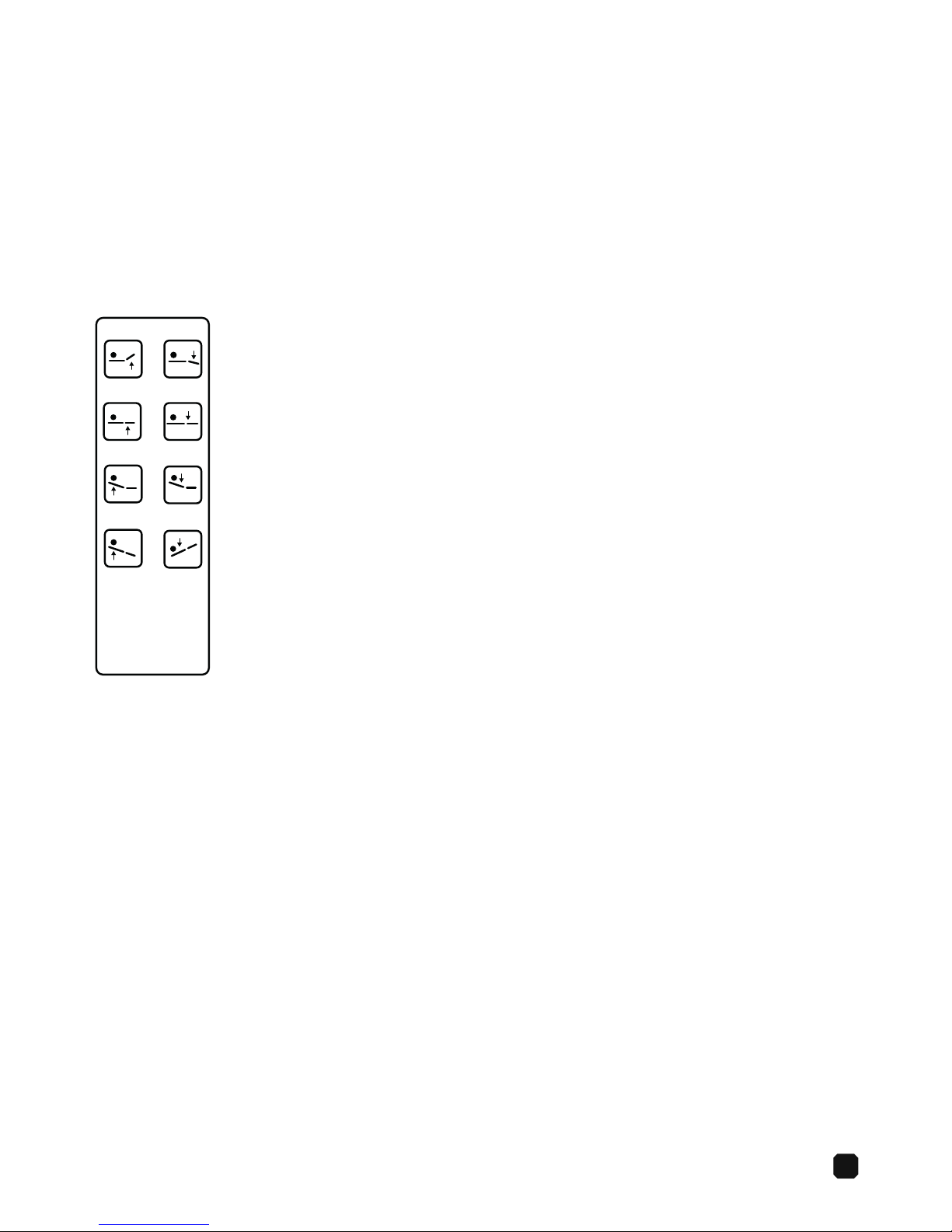
7
Controls
General Operation
The table is operated with the hand control.
Maximum Duty Cycle
2 minutes per 18 minutes
Hand Control
1. Up (seat)
2. Down (seat)
7. Reverse Trendelenburg
8. Trendelenburg
5. Up (back)
6. Down (back)
3. Up (chair)
4. Down (chair)
Emergency Stop
Use the emergency stop if you want to terminate all movements of the table.
• Activate the emergency stop by pushing the emergency stop knob.
• Deactivate by turning the knob to the right. The knob will then jump back to its original position.
The back-up battery charges every time the power cord is connected. The battery turns on automatically when the power
is cut.
Battery Back-Up System

8
Control of Pelvic Position
The chair can be used to rotate the pelvic area, this is described in the following section.
Pelvic Rotation:
Back up and back down
Reverse Trendelenburg and
Trendelenburg
Use the hand control to rotate the table
position.
Hand control buttons:
Use the hand control to lower the table
back mattress below horizontal position.
Use hand control to set the table into desi-
red position.
Control of Standing Position
The table can be placed in a standing position, this is described in the following section. When standing on the footrests
the footrests will hold the weight 551 Lbs, 250kg.
• Remove funnel and its holder, drainage receptacle or other accessory fitted beneath seat cushion before setting the
table in standing position.
• Makes sure the table is in it highest position before setting the table in standing position.
Standing position:
Move back cushion into up-right position,
with the hand control.
Used in Standing Position it is recommended to:
• use both arm rests
• have the footrests as close to the ground as possible, for patient comfort
• distribute the patient weight evenely between the two foot rests
Back up and back down
Reverse Trendelenburg and
Trendelenburg
Hand control buttons:
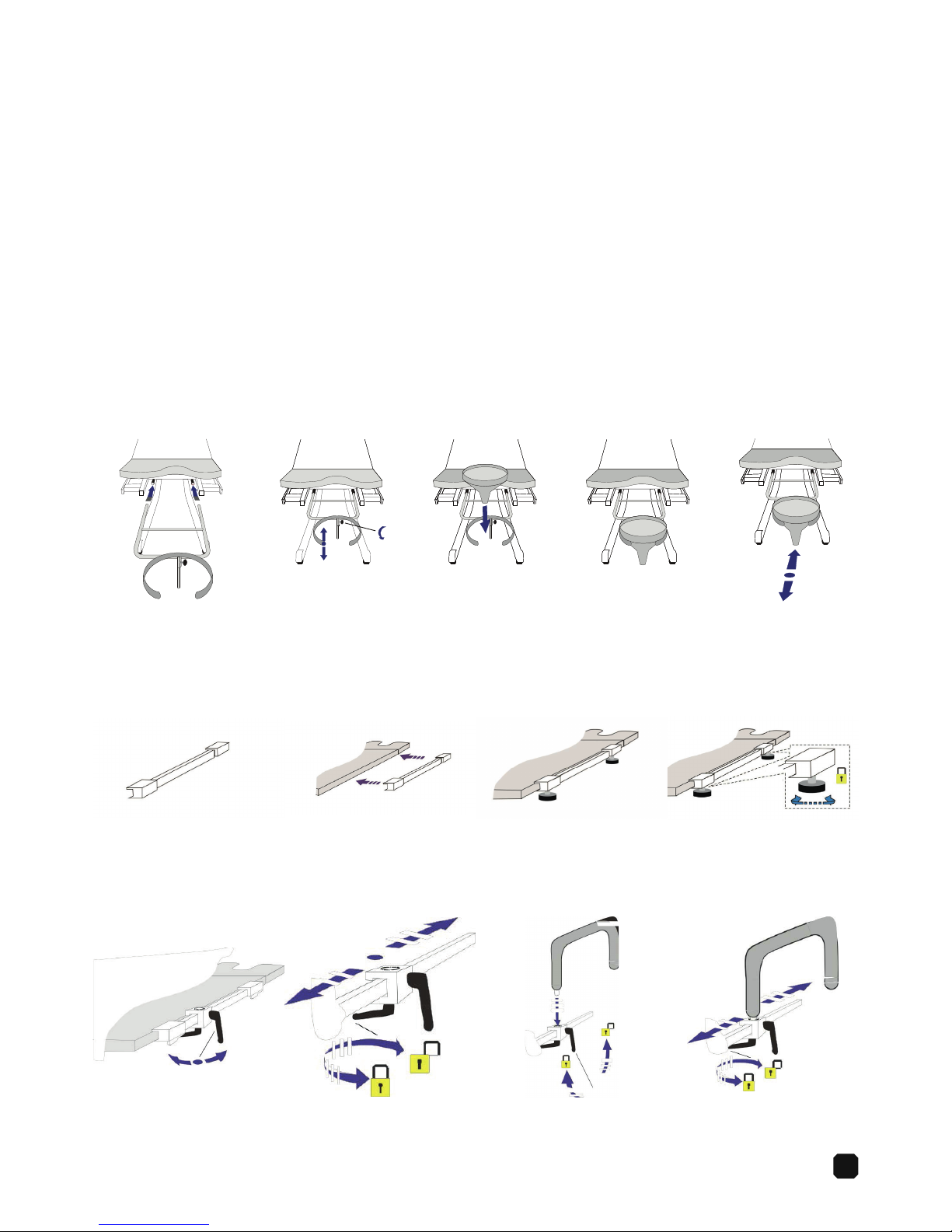
9
Accessories
The 6210 has several accessories, either standard or optional.
These are described in the following sections.
Standard accessories
1 Hand control 524-6356
1 Plastic funnel w/drainage 524-6325-9
1 Arm rest holder 524-6338-4
1 Arm rest 524-6021-6
2 Leg rest holder 525-6338
2 Heavy duty Rissler Leg rest 525-6336
2 Fold up foot rest 525-6331-10
1 Side Rail 524-6329
Plastic funnel drainage — adjustable height
Installation:
1. 4.2. 5.3.
Standard
Detachable side rail — manual lock
Installation:
1. 4.
2. 3.
Standard
Arm rest
Installation:
1. 4.2. 3.
Standard

10
Leg rest holder
Installation (5 mm Allen wrench required):
1. 4.
7.
2.
5. 8.
3.
6.
Leg rest
Installation:
1. 4.2. 3.
Standard
Standard
Foot rest
Installation:
1.
7.
4.
2.
5.
3.
6.
Standard
11
Cleaning
WARNING: Improper, or lack of cleaning after each use of the chair will cause
damage to the appearance and performance.
WARNING: Iodophor type disinfectants (betadine, for example) may stain the
cushions.
WARNING: Prolonged contact with bleach solutions with improper dilution ratios (for)
in any cleaning agent may cause damage to both the appearance and performance.
Maintenance
On a weekly basis, test the device for proper function and inspect cables for cuts and other damage. If in doubt, replace
the relevant parts. The device should be cleaned routinely, as described in the Cleaning section. Additional preventive
maintenance is required according to the Service Manual.
• The unit needs to be unplugged from the wall power before any disinfection.
• Wipe all surfaces after each patient use.
• Please note that it is recommended to remove the seat cushion after each use and to wipe the surfaces below the seat
cushion as well as the back of the seat cushion.
• Clean the chair with a damped cloth and ordinary disinfectants. Follow established protocols for cleaning body fluids
from the chair surfaces.
• Use any of the below listed recommended disinfectants on all surfaces. If they are not available, use any disinfectant
with 1:10 dilution of bleach (5.25% sodium hypochlorite).
Recommended disinfectants:
• PDI Sani-Cloth SANI-CLOTH ® Bleach Wipe: Germicidal Disposable wipe.
• Clorox HealthCare Disinfecting Wipes ®

12
The following cleaning and decontamination materials are known to be compatible with medical devices made of
coated-flexible-foam and UltraFoam materials and rigid formed plastics, including rigid parts reinforced with carbon fiber
or glass fiber:
• Rubbing alcohol (70 % isopropyl alcohol).
• Bleach solution (10 % household-type bleach in cold, cool or warm water).
• General-purpose soap- or detergent-typ cleaners (”409”, etc.) at their normal
manufacturer-recommended strength.
• Detergent cleaners/disinfectants that also contain hydrocarbon solvents such as butyl acetate,
acetone, etc., which can dissolve plastic materials. Such cleaners are sometimes described as being
intended for removal of graffiti, or heavy degreasing.
• Ammonia solutions, which can cause some plastics to turn greenish.
• Detergent-type cleaners at abnormally high concentrations (i.e. concentrated cleaners, intended to
be used in diluted form, that instead are used in undiluted, concentrated form).
• Steam.
• Water or other cleaning materials at temperatures higher than 150°F/66°C.
• Certain grades of “Vesphene” and similar sterilants/cleaners.
• Acids of any kind.
• Rough brushes, aggressive scrubbers, sharp objects, and abrasive materials.
• Iodine solutions, such as “Betadine”.
Avoid the following types of materials for cleaning:
The most common clinical contaminants, i.e. skin oils, blood, urine, vomit, feces, contrast medium, etc., are cleanable
from patient contact surfaces of CFI coated- flexible-foam, UltraFoam and rigid formed plastic products using
general-purpose soap- or detergent-type cleaners (”409”, etc.) at their normal manufacturer-recommended strength. If
needed, rinse with clear water (temperature no higher than 150°F/66°C) to remove gross contamination before applying
the cleaner. Dried contamination may need to be kept moist for an extended period to re-hydrate the contaminant materi-
al before cleaning.
Avoid vigorous scrubbing, stiff brushes, sharp objects such as knives and scrapers, abrasive cleansers, abrasive pads,
steel wool, and similar cleaning methods that are capable of causing mechanical damage. Whenever possible, vomit
and other aggressive contaminants should be wiped or washed off surfaces promptly so as to minimize the likelihood of
permanent chemical-attack effects.
Mattress cleaning instructions

13
Technical Data
Measures and Weights
Model
Video Fluoroscopy Table, 6210, three motors (lift, seat, back).
Power Supply
Power consumption: max 5.8A (120V~), 3.0A (230V~).
Input: 120 V ~ 60 Hz.
230 V ~ 60/50 Hz.
Output: 24VDC (to Motor).
Type of Protection: Class 1, Type B.
Operating Conditions
Temperature +10°C to +40°C (50°F to 104°F).
Humidity 30% – 75%.
Atmospheric pressure 700hPa – 1060hPa.
Storage Conditions/Transportation Requirements
Temperature -40°C to +70°C (-40°F to 158°F).
Humidity 10% – 80%rh.
Including Condensation.
Atmospheric Pressure 700hPa – 1060hPa.
Video Fluoroscopy Table 6210
Seat tilt: 0º to vertical 90º
Back tilt: vertical 90º to -15º trend
Maximum patient weight: 250 kg/551 lbs
Unit Width (max.) Length (max.) Height (min.-max.) Chair weight (max.)
Metric (cm) 115 156 W/O extension
194-205 W/ extension
51-137 224 kg
US (Inches) 45 61 W/O extension
76-81 W/ extension
20-54 493 lbs.
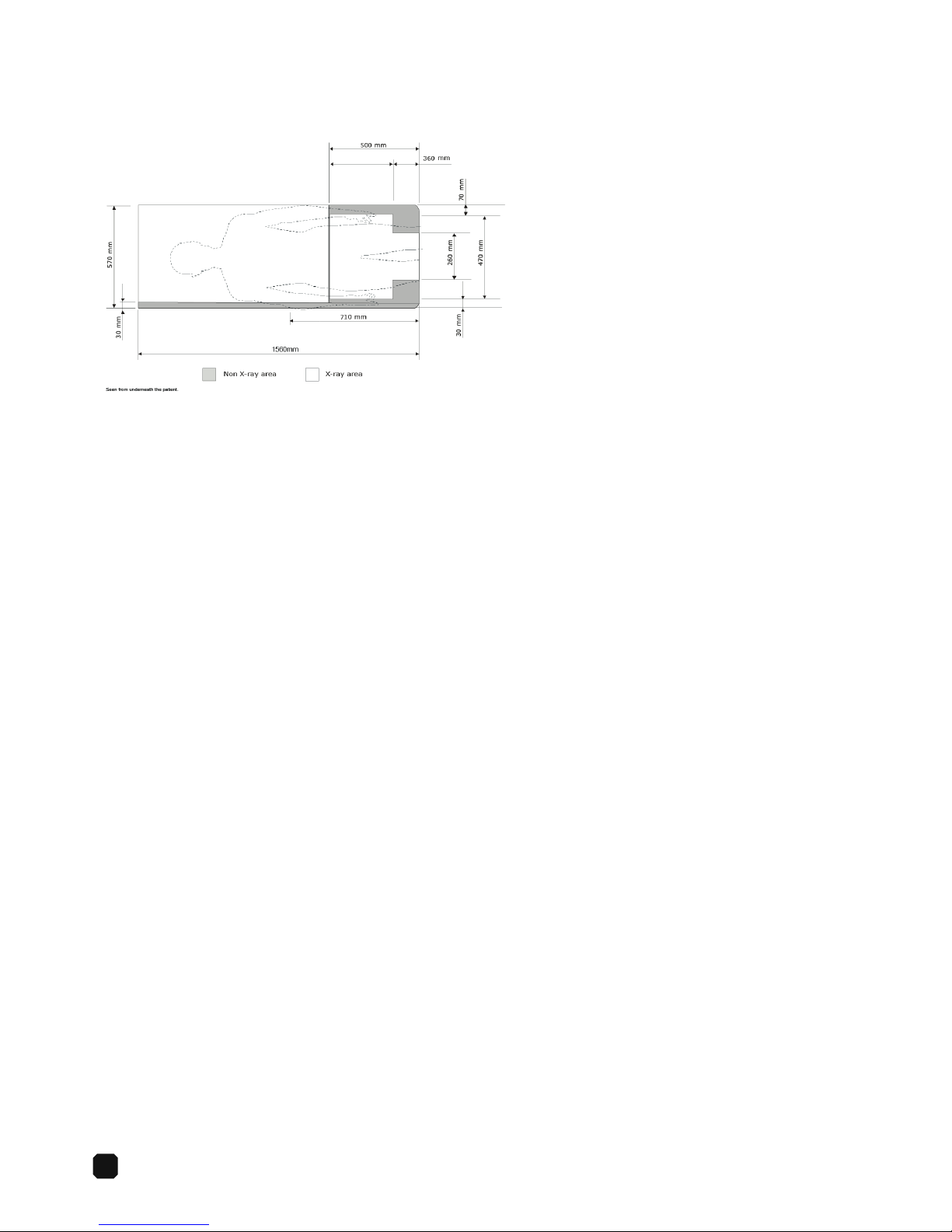
14
Imaging Area
Classification
Degree of protection against electric shock:
• Type B (Body): equipment providing particular degree of protection against electric shock, particularly regarding
allowable leakage current.
Degree of protection against harmful ingress of water:
• IPX6
Degree of safety in presence of inflammable anesthetics:
• The device is not intended for use with flammable anesthetic gases.
Mode of operation:
• Intermittent operation.
Duty Cycle:
• 2 minutes per 18 minutes.
This product has a technical lifetime, which by Sonesta Medical AB is considered to be 10 years. At the time of delivery
the product fulfils the existing regulations and standards, but as all other electro-mechanical products, the Sonesta Video
Fluoroscopy table is subjected to age and wear, and even though the product undergoes regular and prescribed service,
Sonesta Medical AB can not guarantee the product’s safety after the expiry of the technical lifetime. Sonesta Medical
AB recommends that the product is taken out of service after latest 10 years. By Sonesta Medical AB provided spare
parts and service after the expiry of the specified technical lifetime does not mean an extension of Sonesta Medical AB’s
liabilities.
Technical Lifetime

15
Notes

Service Centers
Sweden
Sonesta Medical AB
Tegeluddsvägen 76
SE-115 28 Stockholm
Sweden
Tel: +46 8 50257280
http://www.sonestamedical.se
USA
Sonesta Medical Inc
2 DeBush Avenue unit C3
Middleton, MA 01949
United States
Tel: +1 630-519-3450
http://www.sonestamedical.se
Manufactured by
Sonesta Medical AB
Tegeluddsvägen 76
SE-115 28 Stockholm
Sweden
Tel: +46 8 50257280
http://www.sonestamedical.se/
TF 2017-09-11
SM-6210-IFU-1702
Other manuals for 6210
1
Table of contents
Other Sonesta Medical Equipment manuals