Steiss UTS User manual

USER MANUAL
Universal
Therapy
System
(UTS)
Contents
22
Section Content Page Number
1. The Purpose of this Manual 3
2. Important Safeguards 3
3. Product Description 4
Intended Use 4
General Safety 4
4. Quick Start Guide: 5
Arrival of Product 5
Preparation of Area 5
5. Installation Guide 6
Initial Set Up 6/7
6. Hand Set / System Control 8
7. Maintenance & Troubleshooting 9
8. Cleaning & Disinfection Protocol 10
9. Specication 11
10. EMCRelatedNotications 12/13/14/15/16
11. Storage & Care 17
12. Waste Disposal 18
13. Symbols Used 18
14. Expected Service Life 18
15. Warranty 19
16. Legal Disclaimer 19
17. Service 20
18. Service Notes 21
19. Service Log 22

1.The Purpose of this Manual
33
2. Important Safeguards
INDOOR USE ONLY
DANGER - READ ALL INSTRUCTIONS BEFORE USING THE APPLIANCE
WARNING: To reduce the risk of burns, electrocution,
re or injury to persons:
1. This product should never be left unattended when plugged into a power outlet.
2. Close supervision is necessary when this product is used by, on, or near children
or physically challenged individuals.
3. Only use this product for its intended purpose, as described in this manual.
4. Never use attachments with this system that have not been recommended or
approved by the manufacturer.
5. Never connect this product to a power supply or operate it if:
A. The system shows any signs of having a damaged cord or plug.
B. It does not appear to work properly or makes any abnormal noise.
C. The products has been dropped or damaged, or dropped into water.
Should any of the above be relevant, return the product to a service center for examination and repair.
6. Keep the electrical cord away from heated surfaces, open ames, liquids and sharp objects.
7. Never drop or insert any object into any openings.
8. Do not use outdoors, operate where aerosol (spray) products are being used,
or where oxygen is being administered.
9. ALWAYS disconnect from power supply before opening up the mattress.
10. The product has no user serviceable parts except for fuse replacement.
11. If pain, irritation, numbness, swelling, or redness occurs discontinue use and contact a
healthcare professional.
When using electrical products, especially when children are present,
basic safety precautions should always be followed, including the following:
To reduce the risk of electrocution:
1. Always unplug this product immediately after use.
2. Do not use while bathing.
3. Do not place or store product where it can fall or be pulled
into a tub or sink.
4. Do not place in, or drop into, water or other liquid, unless
following specic manufacturers guidelines.
5. Do not reach for a product that has fallen into water.
Unplug immediately.
This operation manual is mainly focused on the set-up, cleaning and routine maintenance of the
Steiss™ Universal Therapy System (UTS). We recommend that you keep this manual to hand to answer any
questions that may arise that are related to the system.
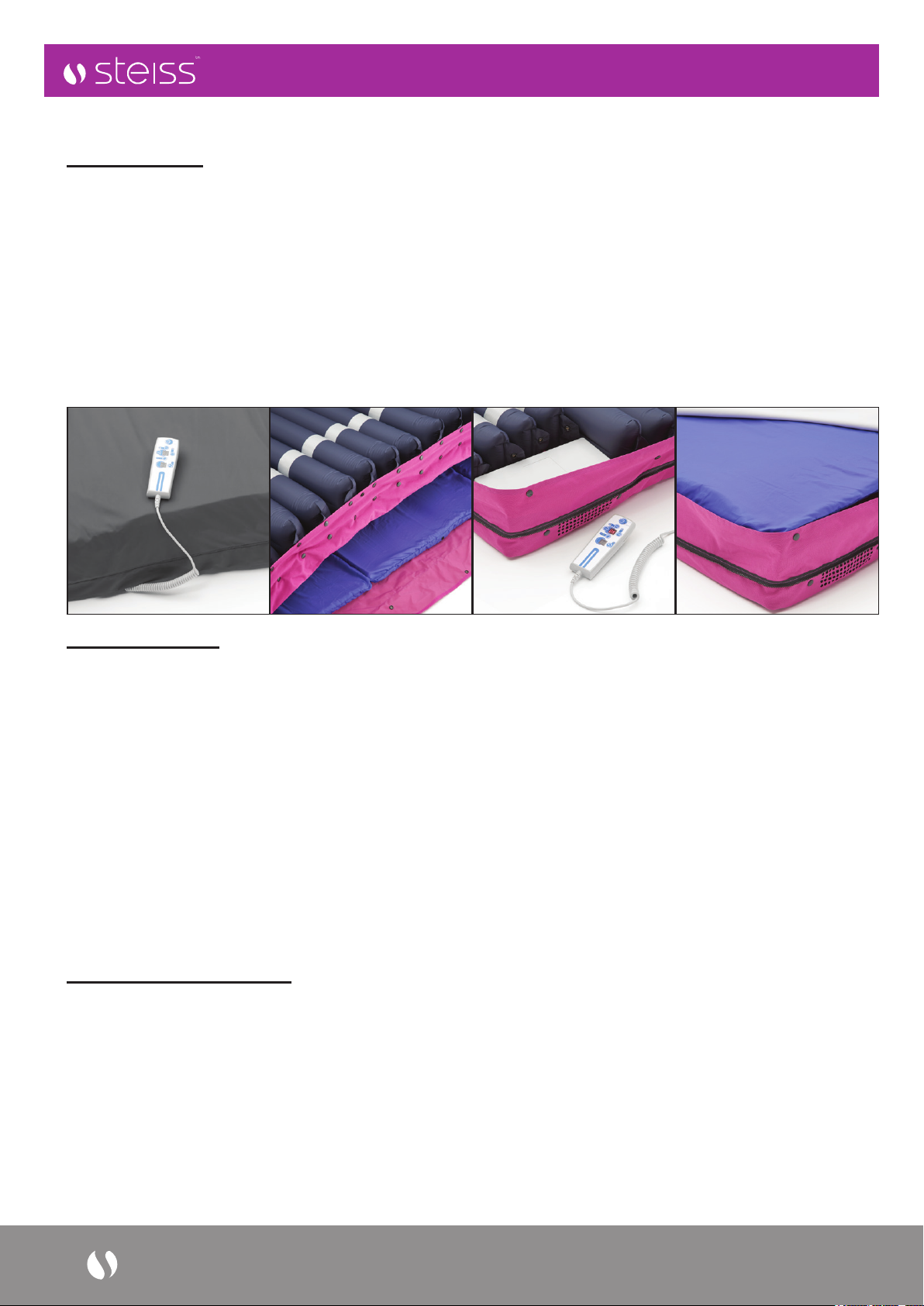
3. Product Description
44
Intended Use
The Steiss™ Universal Therapy System (UTS) is an alternating air replacement system designed to prevent
and treat pressure ulcers. The Steiss™ Universal Therapy System (UTS) works on the principle of actively
encouraging tissue blood ow by gently increasing and decreasing pressure within the support surface over
a 10-minute cycle. This results in the reduction of surface pressures helping to prevent tissue breakdown and
encourage healing.
For added durability, and longer working life, the Steiss™ Universal Therapy System (UTS) has been
constructed using high strength components and premium grade materials. The mattress features a heavy-duty
base layer and a 100% waterproof, multi-stretch vapor permeable PU cover to reduce risks of friction and shear.
General Safety
It is important to read the information in this user manual before you use your
Steiss™ Universal Therapy System (UTS).
Please follow the guidelines below for your added safety and maintaining system performance.
● Maximum patient weight is 175kg and minimum user weight is 30 kg.
● Avoid exposing pump to liquids.
● When cleaning do not use Phenol based substances.
● This Steiss™ Universal Therapy System (UTS) must be used on top of a bed frame.
● Never block the air opening or insert any object into them.
It is at all times the Carers responsibility to ensure that there is adequate/ legal clearance from the top of this
mattress to the top of any side rails tted to the bed.
IntendedUserProle
● Education/Knowledge: 8th grade+
● Must be able to read and understand ‘Westernized Arabic’ numerals when written in Arial font
● Have a basic understanding of hygiene standards
● Have relevant experience in a nursing environment
● Permissible impairments: Except for contraindications
4. Quick Start Guide
55
Arrival of Product
BOX 1:
Air-Alternating mattress, pump and manual
Preparation of Area
1. Place the Steiss™ Universal Therapy System (UTS) directly on top of the bed frame and ensure that the
CPR is at the head of the bed. Secure the mattress using the straps at the base.
CAUTION: When securing the mattress to the bed, please ensure that you are securing
onto the movable parts of the bed and NOT the actual bed frame, as this will obstruct the
mechanical movement and could cause damage to both mattress and bed. The warranty for
both the mattress and the bed may well be invalidated if damage is caused by this means.
NOTE:Whenthemattressisopenedforthersttime,pleasewritetoday’sdate
(with a non-Phenol based pen) in the top white box on the PU cover and add in the ‘same
date’forthefollowingthreeyearsinthebottomwhitebox.Thiswillremindthecareteam
when the annual service is due.
2. Hang the hand set on to the bed rail or mattress platform and plug the power cord into the mains
outlet. Ensure that electrical cables are safe and tidy and are not caught in the bed frame and free
from obstruction. The power LED will be at standby mode with amber light illuminating.
CAUTION: it is important to routinely inspect power cable to ensure it is not obstructing or
causing a tripping hazard. Check the power cable is not under strain or damaged.
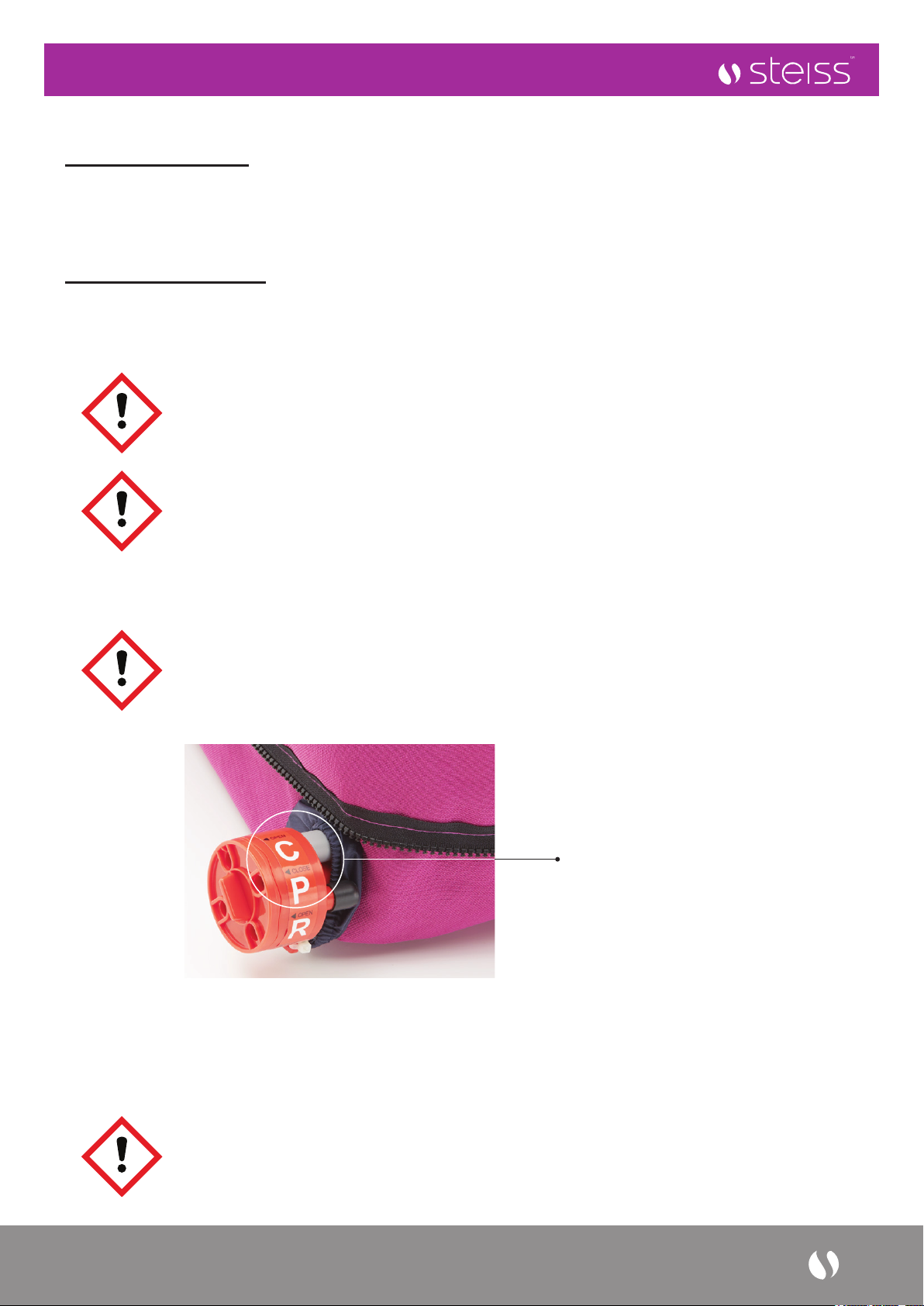
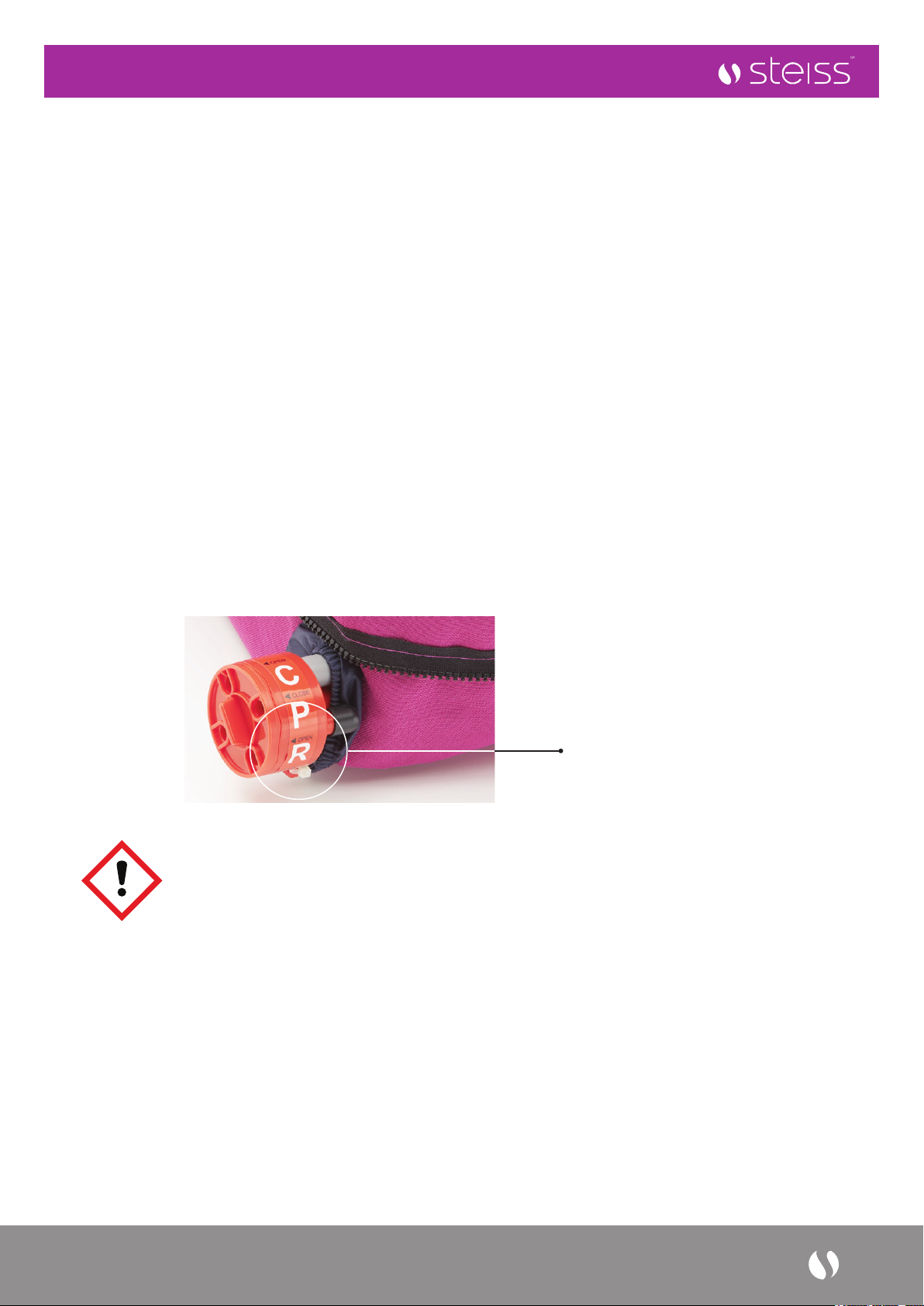
3. Ensure that the CPR valve is set to ‘CLOSE’ position.
4. Turn the power on from the handset (Green LED illuminated)
5. When the Steiss™ Universal Therapy System (UTS) is turned on, the pump will start to inate the mattress
for around 30 minutes in alternating mode. During this ination period the ‘AF’ (Auto Firm) will be displayed
on the LED window to indicate the mattress is not ready to use.
CAUTION: It is important to ensure that no person/ object is lying/ sitting on the mattress
whenitisbeinginated.
Turn the dial
to ‘CLOSE’
position to inate
the air mattress.
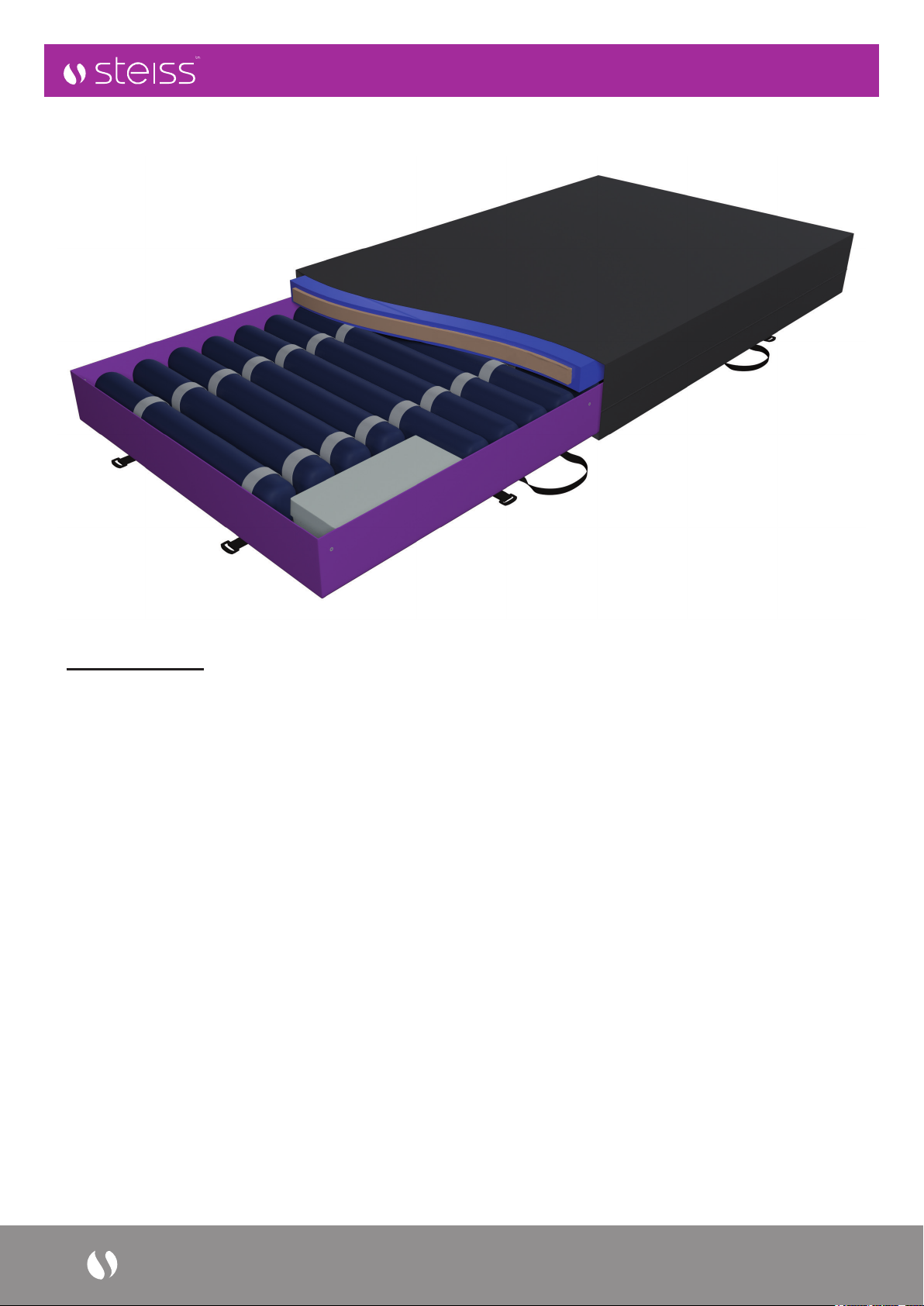
5. Installation Guide
66
Initial Set Up
The UTS mattress denes a new product category within the pressure care mattress range spectrum. A step up
from traditional hybrid system. Allows the user to adopt and customize the system to suit a wider range of care
requirements.
1. STATIC COMFORT MODE
Mattress is fully inated and held in static mode with top surface foam in position underneath top PU outer
cover (mattress is plugged in and switched on in standby mode)
2. DYNAMIC COMFORT MODE
Mattress is fully inated and operated in auto dynamic mode with top surface foam in position underneath.
Top PU outer cover.
3. DYNAMIC MODE (AUTO)
Mattress is fully inated and operated in auto mode with patient in direct contact with alternating cell surface for
maximum reactive therapy. Foam top surface is stored inside the pocket beneath the dynamic cells.
4. DYNAMIC MODE (MANUAL)
Mattress is fully inated and operated in manual dynamic mode with patient in direct contact with Alternating
cell surface (underneath top PU outer cover). Surface pressure is adjusted manually via handset to maximize
patient comfort (constant low-pressure therapy – CLP). This is particularly benecial to hyper sensitive patients
where the benets of the reactive therapy are matched to comfort.
5. Installation Guide
77
When the Steiss™ Universal Therapy System (UTS) is turned on, the pump will start to inate the mattress for
around 30 minutes in alternating mode. During this ination period the ‘AF’ (Auto Firm) will be displayed on LED
window to indicate the mattress is not ready to use.
When ‘MAX’ LED goes off, a bed sheet can be added (NOT tucked under and left to hang free) and a patient can
be placed on the mattress surface and positioned. The audible alarm is disabled during the ination period and will
resume its function when the ‘AF’ LED goes off.
Once the mattress is properly inated, system will go to alternating mode. Press ‘auto’ button to enable auto
detection feature and the system will automatically set to an optimum pressure by patient weight.
The Steiss™ Universal Therapy System (UTS) also allows the care giver a exibility to disable ‘auto’ feature
and switch to manual adjustment, by repeatedly pressing ‘LEVEL’ button to accommodate an individual’s comfort if
needed. Default manual setting will be the level detected during ‘Auto’ mode, or at level 2 if detection is incomplete.
Please note: ‘AUTO FIRM’ and ‘STATIC’ mode is designed to return to ‘ALTERNATE’ mode after
30 minutes of activation.
This Mattress features a cardio Pulmonary Resuscitation (CPR) valve at patient’s right side at the head end.
In the event of cardiac arrest, turn the CPR valves to the ‘OPEN’ position for fast deation of the air mattress
Turn the dial to OPEN position to release air.
IMPORTANT: please ensure that the care staff are trained and familiarized with the mattress and
this function.
CAUTION: During power failure/ outage, the Steiss™ Universal Therapy System (UTS) will stop
functioning and the PF (power failure) alarm codes will be displayed on LED window with audio
alarm. The pump will return to its normal operations when power is resumed.
The design of the system does allow the safe support of a patient even in the case of a power
failure and loss of air from within the mattress.
In the event of product failure, unless the mattress has been ‘set’ to operate as a standard,
non-powered system or altered to be a ‘step-down’ option, return the product to a service center
for examination and repair.
Turn the dial to
OPEN position
to release air.
Attention: If the airow output varies, becomes unstable or stops erratically, it could be caused from
EMC disturbance or an unstable power socket. Be sure to use the device with a stable power supply
or in connection with UPS and repress the power ON button to activate the air replacement system.
6. Hand-Set / System Control
88
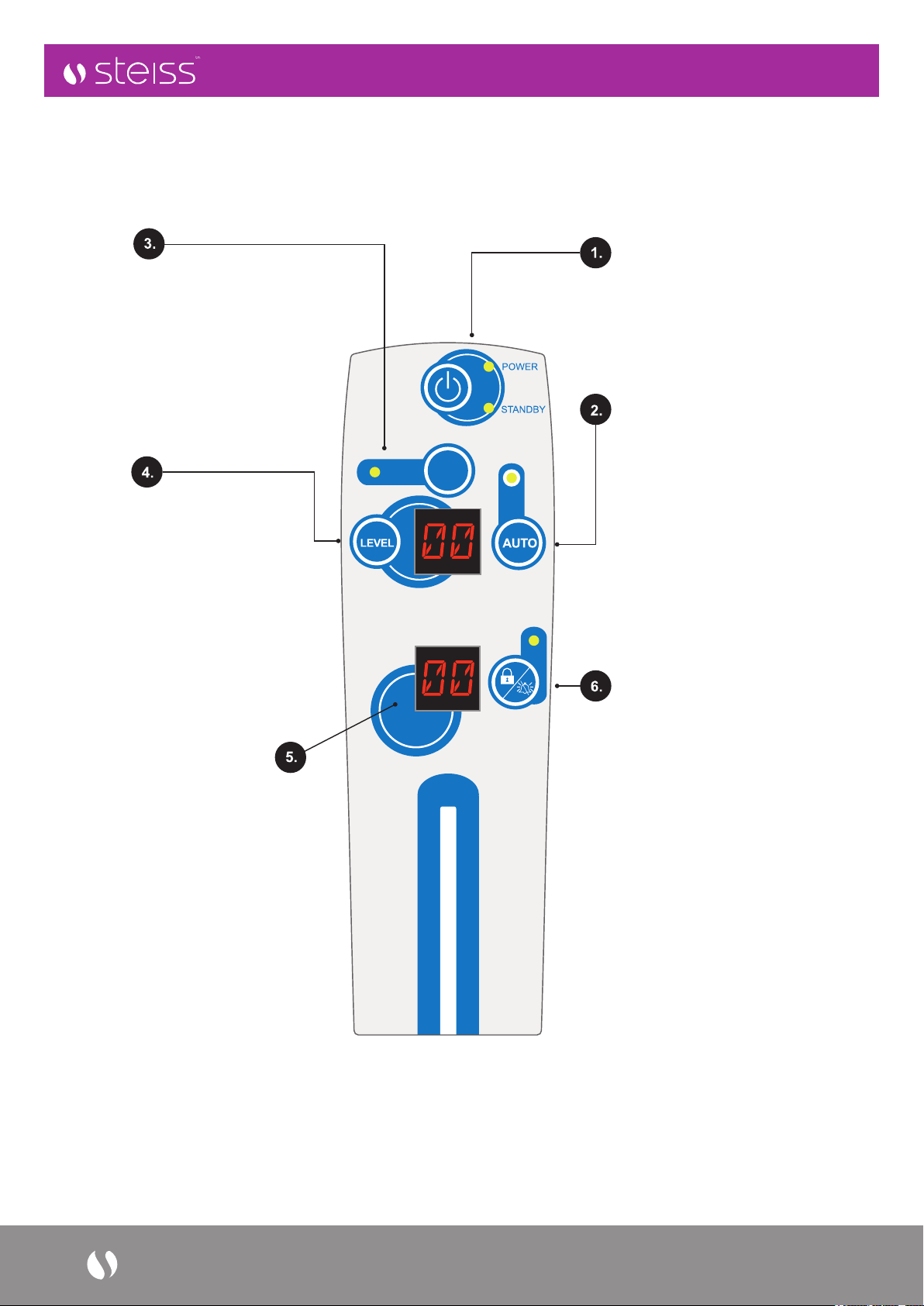
MODE
POWER -
Power On (Green LED),
Standby (Amber LED)
AUTO -
Weight Detection function,
is enabled by press “Auto” button
and will self-adjust the comfort
level by patient’s weight. NOTE:
The “AUTO” detection mode will
be disabled when comfort level is
manually adjusted. Press “AUTO”
again to re-activate the automatic
weight detection when needed.
MODE -
to select Auto Firm (AF), Alternating
(AL) and Static (ST), Therapy Modes
by repeatedly pressing “Mode” button
– the pump is set to alternating mode
by default with “AL” display on LED
window. UTS is programmed to default
back to alternating mode after 30
minutes of usage.
LEVEL -
allows for manual adjustment,
by repeatedly pressing “LEVEL”
button to accommodate an
individual’s comfort if needed.
Default manual setting will be the
level detected during “Auto” mode,
or at Level 2 if Auto detection is
incomplete. Display of “LEVEL”
will automatically go back to
“MODE” when not in operation
for 10 seconds.
ALARM CODES DISPLAY -
When LP (Low Pressure) occurs,
system will alert user with a visual
alarm displayed in LED display
window, and audible alarm will be
triggered 5 minutes after visual alarm.
PF (Power Failure) will be displayed
in LED window with an audio alarm to
indicate a power outage situation.
LOCK/UNLOCK/
ALARM MUTE BUTTON -
allows user to manually lock
(3 seconds) / unlock (3 Seconds)
the control panel to safeguard
against any unwanted attempt to
alter the settings.
Control panel will auto lock
when system is not in operation
for 5 minutes.
Alarm Mute: Press to mute the
audio alarm. The alarm will
re-activate after 20 minutes
if the problem is not resolved.
ALARM
CODE
7. Maintenance & Troubleshooting
99
No daily maintenance is required. It is intended this equipment should only be
serviced by qualied and authorised technical personnel.
Fault/Problem Description Troubleshooting Solution
No indication that the pump is on.
Check Steiss™ Universal Therapy
System (UTS) is connected to the
mains power supply.
Check the power is switched on.
Check for loose connection on plug
and main power is switched on.
Secure plug connection and turn on
power from main.
Check for blown fuses in plug. Replace fuse if blown.
Check if mains socket is faulty. Try another socket
Low Pressure in mattress.
Mattress not inating.
Check Steiss™ Universal Therapy
System (UTS) is connected to the
mains power supply.
Ensure the main power is turned on
and Steiss™ Universal Therapy
System (UTS) is plugged into mains.
Check Steiss™ Universal Therapy
System (UTS) air connections are
tted securely. Ensure airow is
coming out from pump.
Ensure connectors are securely
fastened and reconnect pump air
hoses if loose. Ensure pump is
turned on.
Check CPR is set to CLOSE position
and is not leaking.
Ensure CPR valve is set to CLOSE
position. Replace CPR valve if an air
leak is found.
Check the connector tubes for kinks,
obstructions or damage.
Untwist any kinks, or move any
obstructions.
Check air intake from lter is not
blocked by linen/dust.
Replace with new lter.
Pump controls lock up or ‘freeze’.
Turn off and unplug Steiss™
Universal Therapy System (UTS).
Rest Steiss™ Universal Therapy
System (UTS) for a few seconds and
plug the back in to main and turn on
the pump.
If problem is still not resolved, please contact your Steiss™ sales representative for advice.
CAUTION: Please ensure the Air Blower is used with stable power supply or in connection with UPS.
8. Cleaning & Disinfection Protocol
1010
It is very important to have a strict cross infection, cleaning and disinfection
policy in line with current Hospital/Nursing Home infection control guidelines.
1. Remove the bedding.
2. If necessary, inate the mattress.
3. Ensure that the power unit is off.
4. Unplug the power cord from the wall outlet.
5. Ensure that the underside of the mattress is clear of all sharp objects.
6. Perform one of the following:
• If blood is present, decontaminate the whole mattress product in line
with current hospital or Nursing Home Guidelines.
• If blood is not present, remove any soil from the cover with paper towels.
NOTE: If grossly soiled, the cover should be removed, cleaned and decontaminated.
7. Using a clean sponge or paper towel, wipe down the cover surface and cells with a diluted detergent solution
or recommended cleaner disinfectant or other germicidal detergent solution.
8. Cleaning and disinfection may be carried out on the cover with hand hot water and a neutral detergent or
with a sodium hypochlorite solution (0.1% or 1000 parts per million available chlorine).
9. Alternatively remove the cover and launder, 95o C (203o F), using normal detergents.
It is essential that articles be thoroughly dried after all cleaning procedures and before storage.
10. Perform the following steps to clean the power unit and hose ttings:
Open the system and expose the pump which is housed in the corner at the foot end of the mattress.
- Wipe all controls, chassis and hose ttings with a damp cloth and a mild detergent.
- Using a nylon brush, gently clean all crevices as they can harbor microorganisms.
- Air dry all treated surfaces.
WARNING:
● Switch off the electrical supply to the pump and disconnect the
power cable from the mains before cleaning and inspection.
● Protective clothing should be worn when performing
cleaning procedures.
● Do not use Phenol based cleaning solutions.
All equipment should be inspected. Any item that is
visibly soiled with the patient’s blood or other body
uids should be properly cleaned or removed. It is
recommended that the system is clean regularly and
after each patient use.
In many cases it will be only be necessary to remove
the mattress cover for cleaning. If there is obvious
soiling a complete cleaning or decontamination
will be required.
9. Specication
1111
Model name: Steiss™ Universal Therapy System (UTS)
Size in CM (L x W x H): 200 x 90 x 18 cm
Weight of system: 15kg
Cycle time (min): 10 min
Max user weight: 175kg
Minimum user weight: 30kg
Min/Max pressure: 20 ~ 60 mmHg +/- 6mmHg
Max ow rate: ≥ 6 L/min
Rated voltage: AC100 – 240V / 50Hz-60Hz
Max current: 0.2-0.1A
Fuse rating: T1AH 250V
Protection type: Class II Type BF
Ingress of water protection: IP22
Mode of operation: Continuous
Environment (Temperature): Operation: 15ºC to 35ºC (59ºF to 95ºF)
Storage: 5ºC to 60ºC (41ºF to 140ºF)
Environment (Humididty): Operation: 30% to 75% non-condensing
Storage: 30% to 90% non-condensing
Operation atmospheric pressure range: 700 hPa to 1060 hPa
Operation altitude: -1017 feet to 9,843 feet (-310 metres to 3000 metres)
Test standard: IEC60601-1, IEC60601-1-2 and IEC60601-1-11
Safety standard: CE
Mattress material: Top Cover: Two way stretch, PU laminated Nylon
Base Cover: PVC laminated Polyester
Packaging in CM (L x W x H): 67 x 27 x 98 CM
Item Model Material
Handset HS-NBE ABS
Accessories:
10. EMC - Related Notication
1212
Warning: Medical electrical equipment needs special precautions regarding EMC and needs
to be installed according to the EMC (Electro Magnetic Compatibility) information provided.
Careful consideration of this information is essential when stacking or collocating
equipment and when routing cables and accessories.
Warning: RF mobile communications equipment can effect medical electrical equipment.
Recommended separation distances between portable and mobile RF communications equipment and the
Steiss™ Universal Therapy System (UTS)
The Steiss™ Universal Therapy System (UTS) is intended for use in an electromagnetic environment (for home
healthcare) in which radiated RF disturbances are controlled. The customer or the user of the Steiss™ Universal
Therapy System (UTS) can help prevent electromagnetic interference by maintaining a minimum distance between
portable and mobile RF communications equipment (transmitters) and the Steiss™ Universal Therapy System (UTS)
as recommended below, according to the maximum output power of the communications equipment.
Rated Maximum output
power of transmitter
W
Separation distance according to frequency of transmitter
(metres)
150 kHZ to 80 MHz
d = 1,2 √P
80 MHz to 800 MHz
d = 1,2 √P
800 MHz to 2.5 GHz
d = 2,3 √P
0,01 0,12 0,12 0,23
0,1 0,38 0,38 0,73
1 1,2 1,2 2,3
10 3,8 3,8 7,3
100 12 12 23
For transmitters rated at a maximum output power not listed above, the recommended separation distance din metres
(m) can be estimated using the equation applicable to the frequency of the transmitter, where Pis the maximum output
power rating of the transmitter in watts (W) according to the transmitter manufacturer.
NOTE 1: At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by
absorption and reection from structures, objects, and people.
10. EMC - Related Notication
1313
Manufacturer’sdeclaration–electromagneticemissions
The Steiss™ Universal Therapy System (UTS) is intended for use in the electromagnetic environment (for home
healthcare and professional healthcare) specied below. The customer or the user of the Steiss™ Universal Therapy
System (UTS) should ensure that it is used in such an environment.
Emissions Test Compliance Electromagneticenvironment–guidance
RF Emissions
CISPR 11
Group 1
The Steiss™ Universal Therapy System (UTS) uses RF energy only for
its internal function. Therefore, its RF emissions are very low and are not
likely to cause any interference in nearby electronic equipment.
RF Emissions
CISPR 11
Class B
The Steiss™ Universal Therapy System (UTS) is suitable for use in
all establishments, including domestic establishments and those directly
connected to the public low-voltage power supply network that supplies
buildings used for domestic purposes.
Harmonic Emissions
IEC 61000-3-2
Class A
Voltage Fluctuations
icker emissions
IEC 61000-3-3
Compliance
During test, the wired control box signal display ashes but it could return to normal status.
The device’s essential performance isn’t affected, therefore there is no concern relating to
basic safety. Please refer to the Risk Management Report for details.
10. EMC - Related Notication
1414
Manufacturer’sdeclaration–electromagneticimmunity
Test specications for ENCLOSURE PORT IMMUNITY to RF wireless communications equipment. The M31 Handset
(HS-BE), M31 Handset (HS-NBE) is intended for use in the electromagnetic environment (for home healthcare) specied
below. The customer or the user of the M31 Handset (HS-BE), M31 Handset (HS-NBE) should assure that it is used in such
an environment.
Test
frequency
(MHz)
Band a)
(MHz) Service a) Modulation b)
Maximum
power
(W)
Distance
(m)
IMMUNITY
TEST LEVEL
(V/m)
Compliance
LEVEL
(V/m)
(for home
healthcare
385 380-390 TETRA 400
Pulse
modulation b)
18 Hz
1,8 0,3 27 27
450 430-470
GMRS 460,
FRS 460
FM c)
±5 kHz
deviation
1 kHz sine
2 0,3 28 28
704-787 LTE Band
13, 17
Pulse
modulation b)
217 Hz
0,2 0,3 9 9
800-960
GSM
800/900,
TETRA 800,
iDEN 820,
CDMA 850,
LTE Band 5
Pulse
modulation b)
18 Hz
2 0,3 28 28
1700-1990
GSM 1800;
CDMA 1900;
GSM 1900;
DECT;
LTE Band
1, 3,
4, 25; UMTS
Pulse
modulation b)
217 Hz
2 0,3 28 28
2450 2400-2570
Bluetooth,
WLAN,
802.11 b/g/n,
RFID 2450,
LTE Band 7
Pulse
modulation b)
217 Hz 2 0,3 28 28
5100-5800
WLAN
802.11
a/n
Pulse
modulation b)
217 Hz
0,2 0,3 9 9
NOTE: If necessary to achieve the IMMUNITY TEST LEVEL, the distance between the transmitting antenna and the
ME EQUIPMENT or ME SYSTEM may be reduced to 1 m. The 1 m test distance is permitted by IEC 61000-4-3.
a) For some services, only the uplink frequencies are included.
b) The carrier shall be modulated using a 50 % duty cycle square wave signal.
c) As an alternative to FM modulation, 50 % pulse modulation at 18 Hz may be used because while it does not represent
actual modulation, it would be worst case
710
745
780
810
870
930
1720
1845
1970
5240
5500
5785
10. EMC - Related Notication
1515
Manufacturer’sdeclaration–electromagneticimmunity
The Steiss™ Universal Therapy System (UTS) is intended for use in the electromagnetic environment (for home healthcare)
specied below. The customer or the user of the Steiss™ Universal Therapy System (UTS) should assure that is used in
such and environment.
Immunity test IEC 60601
test level
Compliance
level
Electromagnectic
environment - guidance (home healthcare and
professional healthcare environment).
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
3 Vrms:
0,15 MHz – 80 MHz
6 Vrms:
in ISM and amateur
radio bands between
0,15 MHz and 80 MHz
80 % AM at 1 kHz
10 V/m
80 MHz – 2,7 GHz
80 % AM at 1 kHz
3 Vrms:
0,15 MHz – 80
MHz
6 Vrms:
in ISM and
amateur
radio bands
between
0,15 MHz and
80 MHz
80 % AM at 1
kHz
10 V/m
80 MHz – 2,7
GHz
80 % AM at 1
kHz
Portable and mobile RF communications
equipment should be used no closer to any
part of the Steiss™ Universal Therapy System
(UTS), including cables, than the recommended
separation distance calculated from the equation
applicable to the frequency of the transmitter.
Recommended separation distance
d= 1,2 √P
d= 1,2 √P 80 MHz to 800 MHz
d= 1,2 √P 800 MHz to 2,5 GHz
Where Pis the maximum output power rating
of the transmitter in watts (W) according to
the transmitter manufacturer and d is the
recommended separation distance in
metres (m).
Field strengths from xed RF transmitters, as
determined by an electromagnetic site survey,
should be less than the compliance level in each
frequency range.
Interference may occur in the vicinity of
equipment marked with the following symbol:
NOTE 1: At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by
absorption and reection from structures, objects, and people.
Field strengths from xed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile
radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy.
To assess the electromagnetic environment due to xed RF transmitters, an electromagnetic site survey should be
considered. If the measured eld strength in the location in which the M31 Handset (HS-BE), M31 Handset (HS-NBE) is
used exceeds the applicable RF compliance level above, the M31 Handset (HS-BE), M31 Handset (HS-NBE) should be
observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary,
such as re-orienting or relocating the M31 Handset (HS-BE), M31 Handset (HS-NBE).
Over the frequency range 150 kHz to 80 MHz, eld strengths should be less than 3 V/m.
a)
b)
10. EMC - Related Notication
1616
Manufacturer’sdeclaration–electromagneticimmunity
The Steiss™ Universal Therapy System (UTS) is intended for use in the electromagnetic environment specied
below. The customer or the user of the Steiss™ Universal Therapy System (UTS) should assure that it is used in
such an environment.
Immunity test IEC 60601
test level Compliance level
Electromagnectic
environment - guidance (for home
healthcare and professional healthcare)
Electrostatic
discharge (ESD)
IEC 61000-4-2
Contact:±8 kV
Air±2 kV,±4 kV,±8
kV,±15 kV
Contact:±8 kV
Air±2 kV,±4 kV,±8
kV,±15 kV
Floors should be wood, concrete, or
ceramic tile.
If oors are covered with synthetic
material, the relative humidity should
be at least 30 %.
Electrical fast
transient/burst
IEC 61000-4-4
±2 kV for power
supply lines
±1 kV for input/output
lines
±2 kV for power
supply lines
Not applicable
Mains power quality should be that of
a typical commercial or hospital
environment.
Surge
IEC 61000-4-5
+ 0.5kV, +1kV line(s) to
line(s)
+ 0.5kV, +1kV,+ 2kV
line(s) to earth
+ 0.5kV, +1kV line(s) to
line(s)
Not applicable
Mains power quality should be that
of a typical home healthcare and
professional healthcare environment.
Voltage Dips, short in-
terruptions and voltage
variations on power
supply input lines IEC
61000-4-11
Voltage dips:
0 % UT; 0,5 cycle
0 % UT; 1 cycle
70 % UT; 25/30 cycles
Voltage interruptions:
0 % UT; 250/300 cycle
Voltage dips:
0 % UT; 0,5 cycle
0 % UT; 1 cycle
70 % UT; 25/30 cycles
Voltage interruptions:
0 % UT; 250/300 cycle
Mains power quality should be that
of a typical home healthcare and
professional healthcare environment.
If the user of the Steiss™ Universal
Therapy System (UTS) requires
continued operation during power
mains interruptions, it is recommended
that the Steiss™ Universal Therapy
System (UTS) be powered from an
uninterruptible power supply
or a battery.
Power frequency
(50/60 Hz)
magnetic eld
IEC 61000-4-8
30 A/m
50 Hz or 60 Hz
30 A/m
50 Hz
The Steiss™ Universal Therapy
System (UTS) power frequency
magnetic elds should be at levels
characteristic of a typical location
in a typical home healthcare and
professional healthcare environment.
NOTE: UT is the AC mains voltage prior to application of the test level.
17
11. Storage & Care
1. Ensure the system is clean and free from infection.
2. Check the power cord and plug for abrasions or excessive wear.
Note: Please follow the recommended guidelines when storing.
It is recommended the following guidelines are used whenever this system is being stored or transported
to another location: Temperature limitations: 5°C (41°F) ~ 60°C (140°F) / Relative Humidity: 30% to 90%
3. Carefully place the hand set and power connection lead onto the mattress in readiness for safe
storage within rolled mattress.
4. Disconnect the CPR valve to allow the mattress to deate quickly
5. Starting at the foot end (motor body end) of the mattress, roll the unit up and use the base
mount straps to secure it.
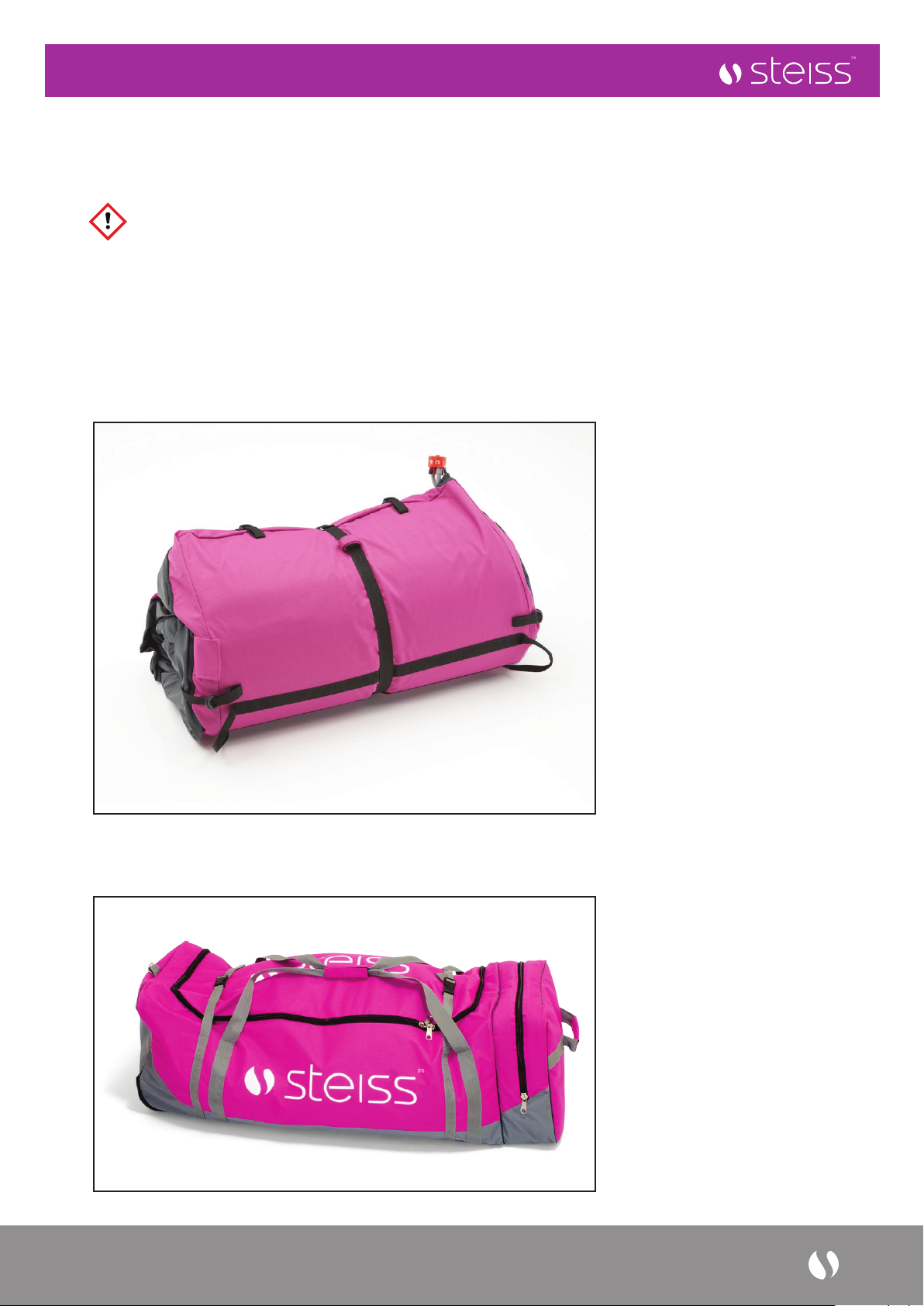
6. Place the Mattress in the Steiss™ Carry bag or Suitable large plastic bag for storage.
18
12. Waste Disposal
This product has been supplied by an environmentally aware manufacturer that
complies with the WEEE (Waste Electrical & Electronic Equipment) directive.
This product may contain substances which could be harmful to the environment if
disposed of in places (landlls) that are not appropriate according to the legislation.
Please be environmentally responsible and recycle this product via your recycling
facility at the end of its product life.
13. Symbols Used
14. Expected Service Life
The Steiss™ Universal Therapy System (UTS) pump has an expected minimum service life of two years.
To maintain the condition of the pump, do ensure that the pump is serviced according to the schedule
recommended by Steiss™.
Do NOT use unapproved accessories or attempt to modify, disassemble or
otherwise misuse the Steiss™ Universal Therapy System (UTS).
Type BF
Protection Against
Electronic Shock
Class II Equipment
Operating Instructions Waste Disposal
Caution, consult
accompanying documents Alternating Current
Manufacturer Interference
~

15. Warranty
• Steiss™ guarantees this equipment to be free from defects in material and workmanship for up to 36
months from the date of delivery to your unit.
• At the manufacturer’s discretion, we agree to service, repair or replace any equipment or part found to be
defective, at no charge.
• This warranty excludes equipment that has been damaged during shipping, or through tampering,
improper maintenance, carelessness, an accident, negligence, or misuse, as well as equipment that has
been altered, repaired or dismantled other than with the manufacturer’s written authorisation and by a
properly qualied technicians following approved procedures.
• In no event shall Steiss™ be liable for any direct, indirect or consequential damage or loss resulting from
the use of this equipment.
• The warranty is non-transferable.
Terms such as ‘Medium Risk’, ‘High Risk’ and ‘Very High Risk’ are descriptions of an individual’s risk level
of developing a pressure sore. (Descriptive Risk Levels). These risk levels are assessed by nurses and as
there may be some variability between nurse measurements/observations, descriptive risk levels should
only be used as a guideline.
Steiss™ uses these Descriptive Risk Levels based on existing market research and internal study to
show the suitability and effectiveness of the pressure care systems provided. Internal and external
research is and will always be ongoing. These Descriptive Risk Levels should not be taken as
prescriptive criteria.
Steiss™ mattresses/cushions should be seen as an aid to care and DO NOT replace the need for good
nursing care and intervention. All Steiss™ products must be used as part of an individualised care plan
which includes proper nursing practices i.e. turning/repositioning, and regular patient skin assessments.
Pressure-relieving equipment alone will not prevent pressure ulcers. Pressure ulcers are multi factorial
and both external and internal factors may cause them to develop. It is up to the professional judgement
of a nurse to assess the risk and develop a care plan which prescribes suitable pressure reducing
equipment and appropriate nursing care. Some pressure sores are inevitable due to falls and longer
periods of immobility. Some sores can developing below the surface of the skin. It may not be visible to
the naked eye and may therefore not become obvious for hours or days after a fall or longer periods of
immobility or injury. For this reason Steiss™ cannot guarantee that the use of the equipment alone will
prevent pressure ulcer formation.
The care giver, operating in accordance with the guide for best clinical practice, will be advising suitable
treatment and just using this mattress manual as a guide only.
16. Legal Disclaimer
A.
B.
C.
D.
E.
19
20
17. Service
RECORD PRODUCT DETAILS
Serial Number
Model Number
Ward or Care Establishment
Deployment date / rst opening date
SERVICE EQUIPMENT
Flow gauge
Manometer
Details of mattress and pump audit proceedure
SERVICE GUIDELINES
General Condition - check these areas: Details:
PU cover, cover welds, screen print and base substrate Look for signs of damage
Mains lead Signs of wear and tear
Pump mechanism Check that they are working properly
Check labelling, decal membrane, buttons and handset Check for excessive wear, uid penetration
and that there is a good button functionality
CPR Mechanism Check it is in good working order
Internal Mattress condition - check these areas:
All T and L connectors Check for wear, strain or damage
Internal assembly tubing Check for wear, strain or damage
Connecting tube Check for wear, strain or damage
Cells condition, including welds Check for wear, strain or damage
Pump Function - Functional calibration
Functional calibration check of pump/mattress system This is a physical test changing modes and
checking cells.
Ensure Dynamic mode and static mode are working in the
pressure range
This is a physical test changing modes and
checking cells.
Ensure power failure alarm and high and low pressure alarms are
working if tted
Turn power off and disconnect hose
separately
Check cell therapeutic pressure Use Manometer (Low mm/hg- high mm/Hg)
on alternation
- Report and replace and defective items
Table of contents
Other Steiss Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual















