TTaabbllee ooff CCoonntteennttss
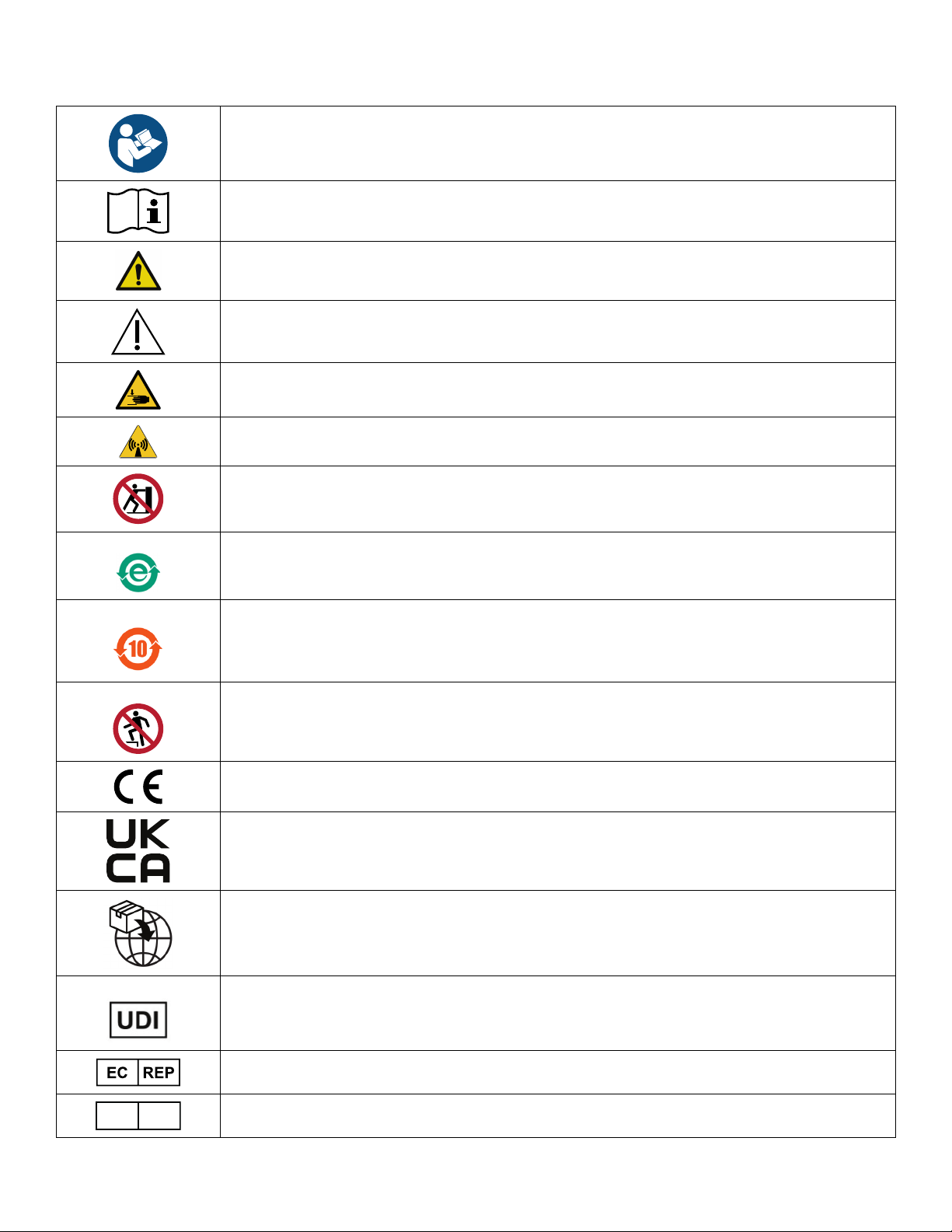
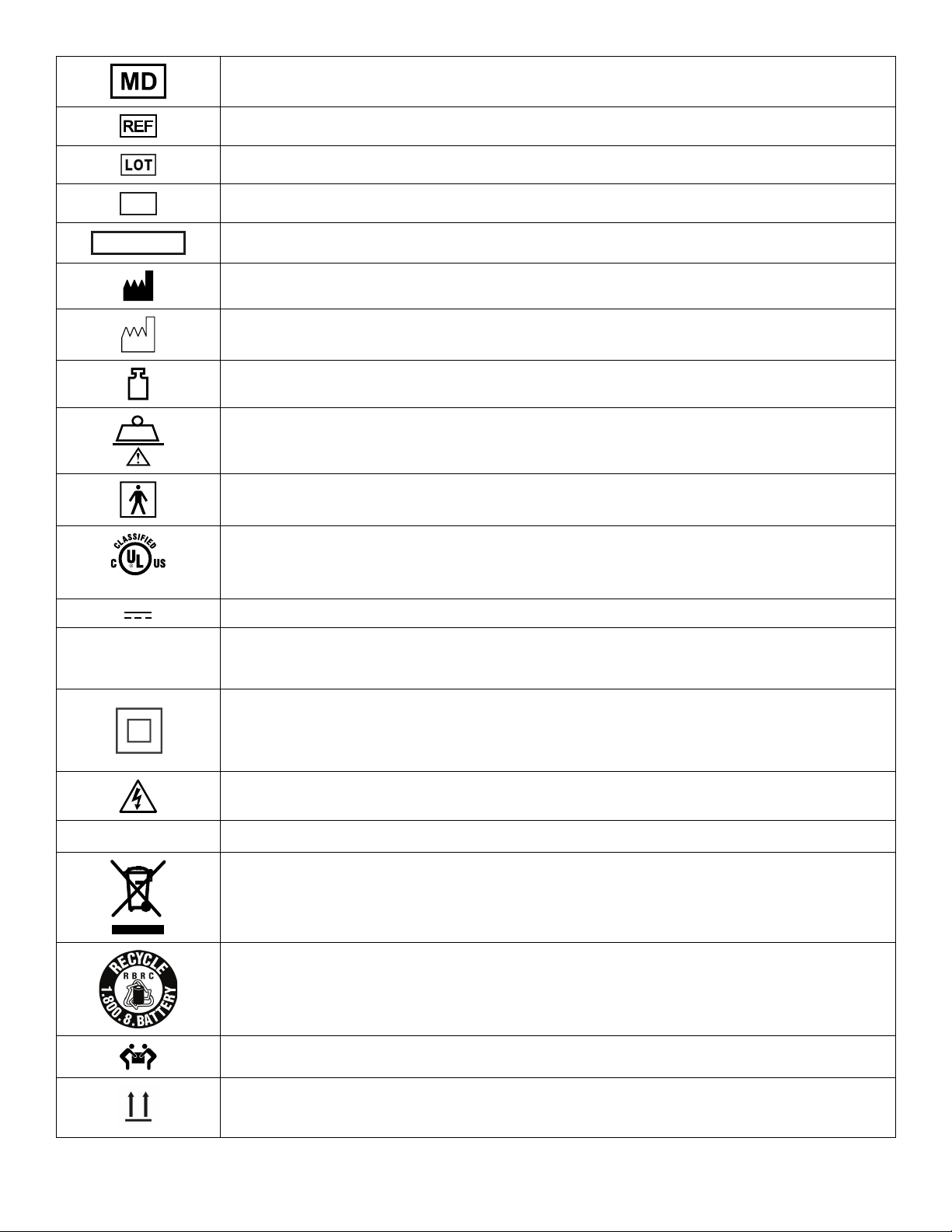
Warning/Caution/Note Definition ..............................................................................................................................3
Summary of safety precautions ................................................................................................................................3
Introduction ...............................................................................................................................................................6
Product description .................................................................................................................................................6
Indications for use...................................................................................................................................................6
Intended users........................................................................................................................................................6
Clinical benefits ......................................................................................................................................................6
Contraindications....................................................................................................................................................7
Expected service life ...............................................................................................................................................7
Disposal/recycle .....................................................................................................................................................7
Specifications - Xpedition ........................................................................................................................................7
European REACH - Xpedition .............................................................................................................................8
Specifications - Alvarium .........................................................................................................................................9
European REACH - Alvarium ............................................................................................................................10
China RoHS - Alvarium.....................................................................................................................................10
Product illustration - Xpedition................................................................................................................................11
Product illustration - Alvarium.................................................................................................................................12
Contact information...............................................................................................................................................12
Serial number location - Xpedition ..........................................................................................................................13
Serial number location - Alvarium...........................................................................................................................13
Date of manufacture..............................................................................................................................................13
Setup.......................................................................................................................................................................14
Operation ................................................................................................................................................................15
Operating guidelines .............................................................................................................................................15
User controls and LED indicators ...........................................................................................................................16
Checking the battery power level............................................................................................................................17
Unfolding the chair ................................................................................................................................................18
Folding the chair ...................................................................................................................................................18
Inserting the battery ..............................................................................................................................................19
Removing the battery from the product ...................................................................................................................19
Storing the battery.................................................................................................................................................20
Charging the battery..............................................................................................................................................20
Electrical power requirements................................................................................................................................21
Charger setup.......................................................................................................................................................21
Securing the charger mounting plate option ............................................................................................................21
Securing the charger to the charger mounting plate option .......................................................................................22
Powering the charger ............................................................................................................................................23
Disconnecting the charger .....................................................................................................................................24
Transferring the patient to the chair ........................................................................................................................24
Proper lifting techniques ........................................................................................................................................24
Securing the patient with the PCS restraint straps....................................................................................................24
Attaching the chest/waist restraint straps............................................................................................................25
Attaching the ankle restraint strap ..........................................................................................................................28
Attaching the head restraint strap option .................................................................................................................29
Transporting the patient on flat surfaces .................................................................................................................29
Transporting the patient down stairs .......................................................................................................................30
Transporting the patient up stairs ...........................................................................................................................31
Applying or releasing a wheel lock..........................................................................................................................32
Raising or lowering the head end flip-up carry handles option...................................................................................32
Supporting the patient’s feet with the footrest option ................................................................................................33
Positioning operators and helpers for additional assistance ......................................................................................33
Attaching the IV hook option ..................................................................................................................................35
Attaching the oxygen bottle holder option................................................................................................................35
Accessories and parts ............................................................................................................................................37
6257-009-001 Rev AF.1 1 EN