1
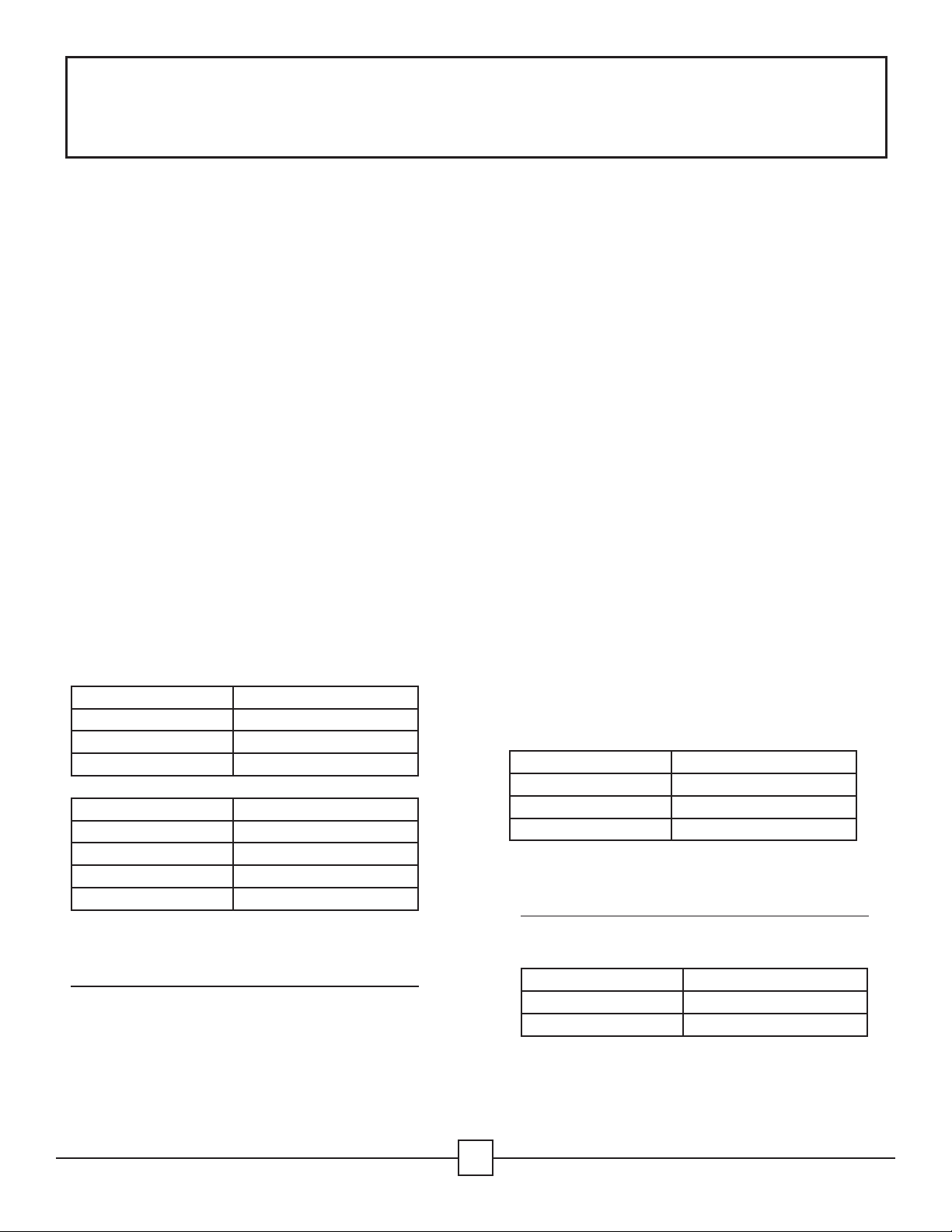
Summit Cassette Model AesculapContainer Model
IN-8823-AE *JN444
IN-2880 *JK444
IN-6105 *JN742
The instructions provided within have been validated by the device manufacturer as being capable of
reprocessing reusable medical devices.
Individual sterilizers, instrument cleanliness, specific loading of instrument trays, types and geometry of
instruments, sterilization containers, filters and wrappings vary at each location.
READ THIS SECTION BEFORE PLACING PRODUCT INTO SERVICE
INDICATIONS FOR USE
InstruSafeInstrument Protection System cassettes are used to organize and protect other medical devices that are sterilized by a
healthcare provider. InstruSafe Instrument Protection System cassettes are intended to allow sterilization of the enclosed medical
devices during these sterilization cycles:
• pre-vacuum steam • STERRAD100NXExpress
• ethylene oxide • STERRAD100NXFlex
• STERRAD100S Standard • STERRADNXStandard
• STERRAD100NXStandard • STERISAMSCOV-PRO, V-PRO 1 and V-PRO maX
The InstruSafe Instrument Protection System cassettes are intended to be used in conjunction with a legally marketed wrap, Aesculap®
rigid container or Genesis™ rigid container. The InstruSafe Instrument Protection System cassettes are not intended on their own to
maintain sterility. A full list of device models is provided in Appendix A.
Device Description:
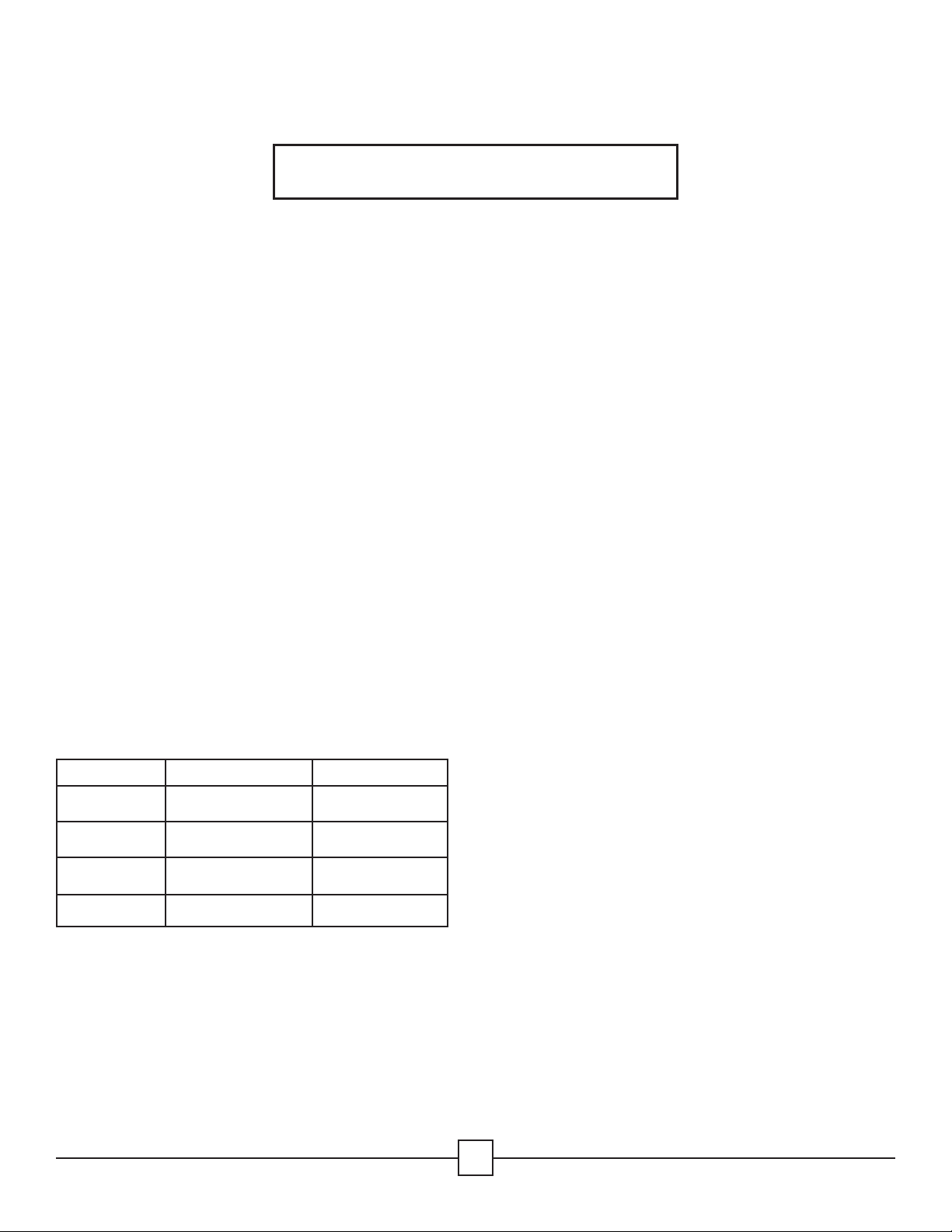
Summit Medical InstruSafe Instrument Protection Systems are cassettes/trays used to enclose and hold surgical instruments and
instrument accessories in an organized manner during the sterilization process and subsequent storage and transportation. The
cassettes/trays do not have direct patient contact. The cassettes/trays by themselves do not maintain sterility. The cassettes/trays are
dierent sizes of the same basic configuration: a rectangular base with latchable cover. The cassettes/trays have perforations to allow
sterilant penetration. The cassettes/trays contain silicone inserts in the base and/or cover to hold, organize and protect the surgical
instruments within the cassette/tray during the sterilization process and subsequent storage and transportation.
Sterilization Methods and Configurations:
The following sterilization trays were validated with the corresponding rigid containers:
» Ethylene Oxide (EO):
Preconditioning Parameters:
Temperature: 131°F (55°C)
Relative Humidity: 70 ± 15%
Time: 1 hour
Sterilization Parameter:
Temperature: 131°F (55°C)
Minimum Exposure Time: 120 minutes
Minimum Aeration Time: 12 hours
*Validated by Summit Medical for use in steam prevacuum sterilizers
ONLY operating at 270°F (132°C) for 4 minutes exposure time.
Consult container instructions to ensure that contents do not exceed
the sterilization containers intended load claims.
*Validated by Summit Medical for use in ethylene oxide sterilizers
ONLY operating at 131°F (55°C). Consult container instructions to
ensure that contents do not exceed the sterilization containers
intended load claims.
» STERRAD100S Standard Cycle:
*Validated by Summit Medical for use in STERRAD 100S Standard
Cycle ONLY. Consult container instructions to ensure that contents
do not exceed the sterilization containers intended load claims.
» Autoclave Sterilization Parameter:
Cycle: Pre-vacuum
Temperature: 270°F (132°C)
Exposure Time: 4 minutes
Minimum Dry Time: 30 minutes
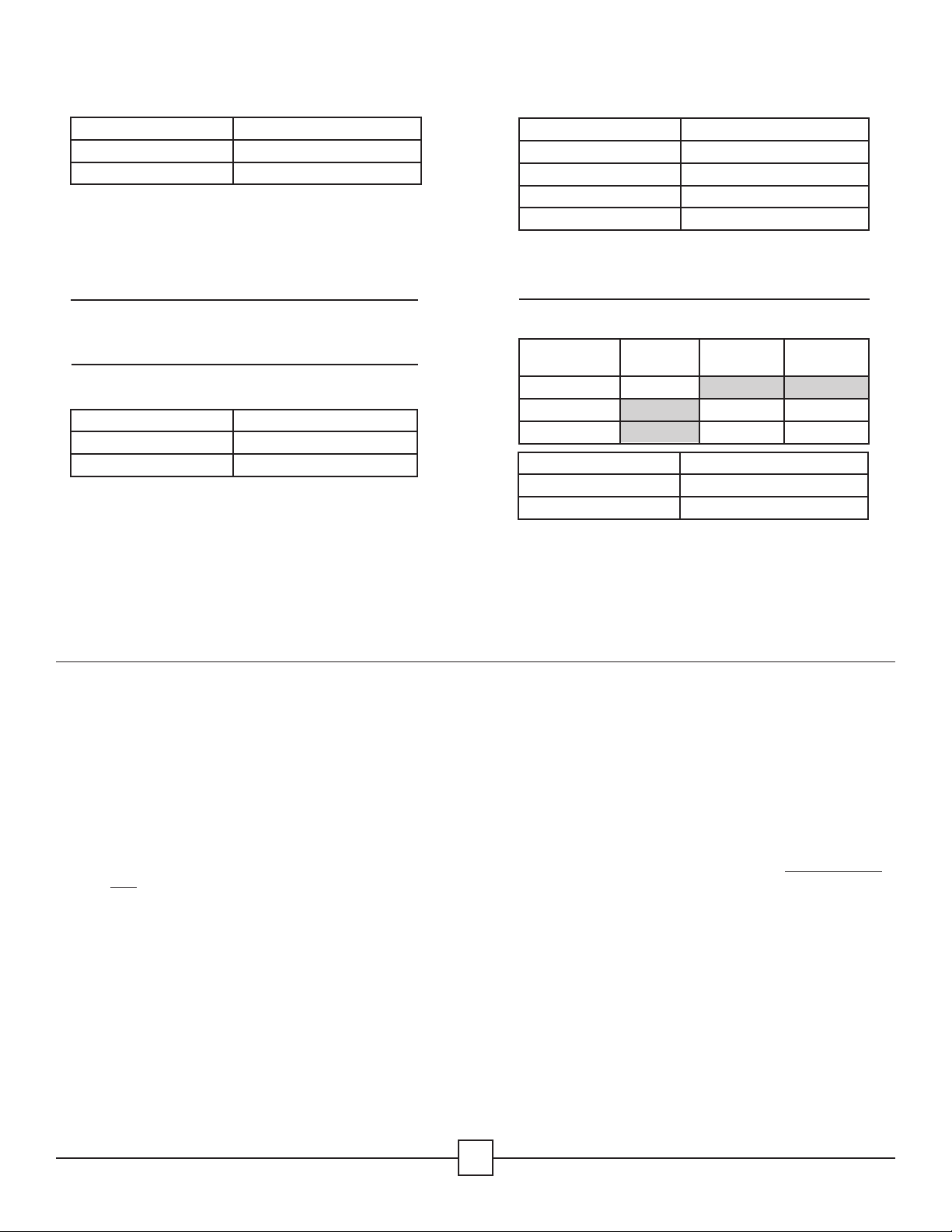
Summit Cassette Model Genesis™ Container Model
IN-2681 *CD2-10-CDL
IN-0000 *CD2-10-CDL
IN-8613 *CD2-10-CDL
IN-6105 *CD2-10-CDL
Summit Cassette Model Genesis™ Container Model
IN-8823 *CD3-9CDL
IN-2880 *CD6-6C
IN-6105 *CD5-61C
Summit Cassette Model AesculapContainer Model
IN-8823-AE *JM444
IN-6105 *JM440