Sunbeam CMS50DL User manual

• Explosive hazard - DO NOT use this device in environment with
inammable gas such as anesthetic.
• DO NOT use this device during an MRI or CT scan, as the induced current
may cause burns.
• DO NOT take the information displayed on this device as the sole basis
for clinical diagnosis. This device is not intended as a replacement for
advice from a physician or medical professional.
• This device is NOT recommended for continuous monitoring.
• DO NOT use on a ngertip that has scar tissue or or that has any other
skin disorder as this may affect the measurement accuracy.
• DO NOT stare directly into the nger chamber of this device while it is
turned on as the infrared light may be damaging to the eyes.
• This device contains silicone, PVC, TPU, TPE and ABS materials, whose
biocompatibility has been tested in accordance with the requirements
in ISO 10993-1, and it has passed the recommended biocompatibility
test. Anyone with allergies to silicone, PVC, TPU, TPE or ABS should not
use this device.
• DO NOT use the included lanyard if you are allergic to nylon/polyester
material.
• DO NOT wrap the lanyard around the neck to avoid an accident.
• This device should be kept out of the reach of children/pets. When
not in use, store this device in a dry room and protect against extreme
moisture, heat, lint, dust and direct sunlight. Never place any heavy
objects on the pulse oximeter.
• DO NOT throw batteries into re.
• DO NOT discard in household trash.
• Only use recommended batteries.
• DO NOT use rechargeable batteries.
• DO NOT drop, disassemble or modify this device.
• DO NOT use this device if you think it is damaged or notice anything
unusual.
• This device comprises sensitive components and must be treated with
caution. Observe the cleaning, maintenance and operating conditions
described in this INSTRUCTION MANUAL.
• DO NOT perform service or maintenance while this device is in use.
• DO NOT touch the battery and the person at the same time.
• DO NOT use this device if it is damaged, degraded or loosened in any way.
• Continuous use of a damaged device may cause injury, improper
results, or serious danger.
• This device needs to be used and serviced in accordance with the
information provided in this INSTRUCTION MANUAL.
OVERVIEW
The oxygen saturation is the percentage of HbO2in the total Hb in the blood,
so-called the O2concentration in the blood, it is an important physiological
parameter for the respiratory and circulatory system.A number of diseases
related to the respiratory system may cause a decrease of SpO2in the blood.
APPLIED RANGE
This Pulse Oximeter is a non-invasive device intended for the spot-check
of arterial hemoglobin (SpO2) and the pulse rate of adults in home use
environments.This device is not intended for continuous monitoring. Solely for
use with sporting and aviation activities. Intended to monitor heart rate
during exercise.
ENVIRONMENTAL REQUIREMENTS
• Storage Environment
a) Temperature: -40°F - 140°F (-40°C - 60°C)
b) Relative humidity: ≤ 95%
c) Atmospheric pressure: 500 hPa - 1060 hPa
• Operation Environment
a) Temperature: 50°F - 104°F (10°C - 40°C)
b) Relative Humidity: ≤ 75%
c) Atmospheric pressure: 700 hPa - 1060 hPa
NOTE: BEFORE YOU BEGIN, WARM THE HANDS AND FINGERS BY
RUBBING THEM TOGETHER.
• Low blood perfusion can affect oximeter readings.
• 99% of reading errors are caused by cold extremities.
• Keep hand and nger still as movement may affect the reading.
• Allow at least 30 seconds for the measurement to stabilize.
• Nail polish and articial ngernails may cause false or no results.
Principle of this Pulse Oximeter is as follows: An experience formula of
data process is established taking use of Lambert Beer Law according to
Spectrum Absorption Characteristics of Reductive Hemoglobin (Hb) and
Oxyhemoglobin (Hb02) in glow & near-infrared zones. Operation principle of
the device is: Photoelectric Oxyhemoglobin Inspection Technology is adopted
in accordance with Capacity Pulse Scanning & Recording Technology, so that
two beams of different wavelength of lights can be focused onto human nail
tip through perspective clamp nger-type sensor. Then measured signal can
be obtained by a photosensitive element, information acquired through
which will be shown on screen through treatment in electronic circuits and
microprocessor.
1. Slide open the back cover and install 2 AAA batteries as shown.To avoid
damage, make sure the batteries align with the “+” and “–” symbols.
2. Replace the back cover.
Figure 4.
Attaching the connector loop
1. Thread the thin connector loop through the hole.
2. Insert the quick-release buckle through the loop.
3. Slide the lanyard clip into the quick-release buckle.
1. Gently squeeze to open the nger chamber.
2. Place nger fully into the chamber.
3. Press the ON button.
Fingertip Pulse Oximeter
Instruction Manual
Figure 1.
Operating principle
AAA
AAA
Figure 2. Fingertip Pulse Oximeter
AAA
AAA
AAA
AAA
Figure 5.
Put nger in position
WARNINGS
PRINCIPLE
TECHNICAL DATA
READ AND SAVE THESE INSTRUCTIONS. PLEASE READ THIS
INSTRUCTION MANUAL BEFORE USING THIS DEVICE. IF THE
USAGE IS NOT FULLY UNDERSTOOD, PLEASE DO NOT USE THIS
PULSE OXIMETER.
ATTENTION
• Use and store this device according the ENVIRONMENTAL
REQUIREMENTS section of this INSTRUCTION MANUAL.
• When the ambient temperature of the device changes too much, such
as when moving from one room at a lower temperature, to another
room at a higher temperature, allow the device to remain in the room
for 30 minutes before using.
• DO NOT use if this device is splashed by or immersed in water.
• High temperature, high pressure, gas sterilizing or immersion
disinfection for this device is not permitted. Refer to the CLEANING &
DISINFECTING section of this INSTRUCTION MANUAL.
• This device may not be suitable for all users. Measurement results
are for reference only. Contact your physician if you have or suspect a
medical condition.
• Data averaging and signal processing have a delay in the upgrade of
SpO2 data values. When the data update period is less than 30 seconds,
the time for obtaining dynamic average values will increase.
• DO NOT use external coloring agents (such as nail polish, articial nails,
or colored skin care products) on the test nger as this may affect the
measurement accuracy.
• Measurement accuracy may be impacted if the test nger:
-Is too cold. Warm the test nger up before using the device.
-Is too thin. Trying using a bigger nger such as the thumb or middle nger.
-Has a long ngernail. Trim the ngernail or switch to a nger with a
short nail.
• Refer to Figure 5 in this INSTRUCTION MANUAL for correct nger
position to use this device. Improper nger position may cause
inaccurate results.
• The light between the photoelectric receiving tube and the light-
emitting tube of this device must pass through the subject’s arteriole.
Make sure the optical path is free from any optical obstacles like
rubberized fabric, to avoid inaccurate results.
• Excessive ambient light may affect the measured results, such
as surgical light (especially xenon light sources), bilirubin lamp,
uorescent lamp, infrared heater and direct sunlight, etc. In order to
prevent interference from ambient light, make sure to place the sensor
properly and cover the sensor with opaque material.
• This device should not be placed on a limb with a blood pressure cuff,
arterial ductus or intraluminal tube.
• The measured value may be inaccurate during debrillation and in
a short period after debrillation, as it does not have a debrillation
function.
• This device has been calibrated before leaving factory.
• This device is calibrated to display functional oxygen saturation.
CLINICAL RESTRICTION
A. As the measurement is taken on the basis of arteriole pulse, substantial
pulsating blood ow of the user is required. For a user with weak pulse
due to shock, low ambient/body temperature, major bleeding, or use
of vascular contracting drug, the SpO2will decrease. In this case, the
measurement will be more sensitive to interference.
B. The measurement will be inuenced by intravascular staining agents (such
as indocyanine green or methylene blue), skin pigmentation.
C. The measured value may be seemingly normal for a user who has anemia
or dysfunctional hemoglobin (such as carboxyhaemoglobin (COHb),
methaemoglobin (MetHb) and sulfhaemoglobin (SuHb), however the user
may appear hypoxia. It is recommended to perform further assessment
according the clinical situation and symptoms.
D. Pulse oxygen only has a reference meaning for anemia and toxic hypoxia,
as some severe anemia patients still show better pulse oxygen measured
values.
E. Contraindication: no
FINGERTIP PULSE OXIMETER COMPONENTS
INSTALLING BATTERIES IN YOUR PULSE OXIMETER
ATTACHING THE LANYARD TO THE PULSE OXIMETER
MEASURING PULSE RATE AND BLOOD OXYGEN LEVELS
Optical sensor
Red light Wavelength: about 660 nm, optical output power: < 6.65 mW
Infrared light Wavelength: about 905 nm, optical output power: < 6.75 mW
Safety class Internally powered equipment, type BF applied part
International
Protection IP22
Working voltage DC 2.6 V - 3.6 V
Working current ≤ 25 mA
Power supply DC 3V (2 x AAA batteries)
Battery life Two batteries can work continually for approximately 24 hours
Dimension 2.2”(L) x 1.2”(W) x 1.3”(H)
57mm(L) x 31mm(W) x 32mm(H)
Weight About 1.8oz /51g (including batteries)
If you have any problems, please do not contact the store.
Contact our customer service at 1-877-383-6399
(8:30 am - 5:00 pm EST) Monday - Friday
or contact us at
Our customer service will be happy to assist you.
© 2021 Sunbeam Products, Inc.All rights reserved.
Distributed by Star Elite Inc., Montreal, Canada H3B 3X9.
SE004-071421
Fingertip Pulse Oximeter Model CMS50DL
Printed in China
Model : CMS50DL
Item: 16979 Figure 3.
Batteries Installation
Power Button
SpO² Display
Battery Level Indicator
Pulse Rate BPM (beats per minute)
Pulse Rate Bar Graph

• Explosive hazard - DO NOT use this device in environment with
inammable gas such as anesthetic.
• DO NOT use this device during an MRI or CT scan, as the induced current
may cause burns.
• DO NOT take the information displayed on this device as the sole basis
for clinical diagnosis. This device is not intended as a replacement for
advice from a physician or medical professional.
• This device is NOT recommended for continuous monitoring.
• DO NOT use on a ngertip that has scar tissue or or that has any other
skin disorder as this may affect the measurement accuracy.
• DO NOT stare directly into the nger chamber of this device while it is
turned on as the infrared light may be damaging to the eyes.
• This device contains silicone, PVC, TPU, TPE and ABS materials, whose
biocompatibility has been tested in accordance with the requirements
in ISO 10993-1, and it has passed the recommended biocompatibility
test. Anyone with allergies to silicone, PVC, TPU, TPE or ABS should not
use this device.
• DO NOT use the included lanyard if you are allergic to nylon/polyester
material.
• DO NOT wrap the lanyard around the neck to avoid an accident.
• This device should be kept out of the reach of children/pets. When
not in use, store this device in a dry room and protect against extreme
moisture, heat, lint, dust and direct sunlight. Never place any heavy
objects on the pulse oximeter.
• DO NOT throw batteries into re.
• DO NOT discard in household trash.
• Only use recommended batteries.
• DO NOT use rechargeable batteries.
• DO NOT drop, disassemble or modify this device.
• DO NOT use this device if you think it is damaged or notice anything
unusual.
• This device comprises sensitive components and must be treated with
caution. Observe the cleaning, maintenance and operating conditions
described in this INSTRUCTION MANUAL.
• DO NOT perform service or maintenance while this device is in use.
• DO NOT touch the battery and the person at the same time.
• DO NOT use this device if it is damaged, degraded or loosened in any way.
• Continuous use of a damaged device may cause injury, improper
results, or serious danger.
• This device needs to be used and serviced in accordance with the
information provided in this INSTRUCTION MANUAL.
OVERVIEW
The oxygen saturation is the percentage of HbO2in the total Hb in the blood,
so-called the O2concentration in the blood, it is an important physiological
parameter for the respiratory and circulatory system.A number of diseases
related to the respiratory system may cause a decrease of SpO2in the blood.
APPLIED RANGE
This Pulse Oximeter is a non-invasive device intended for the spot-check
of arterial hemoglobin (SpO2) and the pulse rate of adults in home use
environments.This device is not intended for continuous monitoring. Solely for
use with sporting and aviation activities. Intended to monitor heart rate
during exercise.
ENVIRONMENTAL REQUIREMENTS
• Storage Environment
a) Temperature: -40°F - 140°F (-40°C - 60°C)
b) Relative humidity: ≤ 95%
c) Atmospheric pressure: 500 hPa - 1060 hPa
• Operation Environment
a) Temperature: 50°F - 104°F (10°C - 40°C)
b) Relative Humidity: ≤ 75%
c) Atmospheric pressure: 700 hPa - 1060 hPa
NOTE: BEFORE YOU BEGIN, WARM THE HANDS AND FINGERS BY
RUBBING THEM TOGETHER.
• Low blood perfusion can affect oximeter readings.
• 99% of reading errors are caused by cold extremities.
• Keep hand and nger still as movement may affect the reading.
• Allow at least 30 seconds for the measurement to stabilize.
• Nail polish and articial ngernails may cause false or no results.
Principle of this Pulse Oximeter is as follows: An experience formula of
data process is established taking use of Lambert Beer Law according to
Spectrum Absorption Characteristics of Reductive Hemoglobin (Hb) and
Oxyhemoglobin (Hb02) in glow & near-infrared zones. Operation principle of
the device is: Photoelectric Oxyhemoglobin Inspection Technology is adopted
in accordance with Capacity Pulse Scanning & Recording Technology, so that
two beams of different wavelength of lights can be focused onto human nail
tip through perspective clamp nger-type sensor. Then measured signal can
be obtained by a photosensitive element, information acquired through
which will be shown on screen through treatment in electronic circuits and
microprocessor.
1. Slide open the back cover and install 2 AAA batteries as shown.To avoid
damage, make sure the batteries align with the “+” and “–” symbols.
2. Replace the back cover.
Figure 4.
Attaching the connector loop
1. Thread the thin connector loop through the hole.
2. Insert the quick-release buckle through the loop.
3. Slide the lanyard clip into the quick-release buckle.
1. Gently squeeze to open the nger chamber.
2. Place nger fully into the chamber.
3. Press the ON button.
Fingertip Pulse Oximeter
Instruction Manual
Figure 1.
Operating principle
AAA
AAA
Figure 2. Fingertip Pulse Oximeter
AAA
AAA
AAA
AAA
Figure 5.
Put nger in position
WARNINGS
PRINCIPLE
TECHNICAL DATA
READ AND SAVE THESE INSTRUCTIONS. PLEASE READ THIS
INSTRUCTION MANUAL BEFORE USING THIS DEVICE. IF THE
USAGE IS NOT FULLY UNDERSTOOD, PLEASE DO NOT USE THIS
PULSE OXIMETER.
ATTENTION
• Use and store this device according the ENVIRONMENTAL
REQUIREMENTS section of this INSTRUCTION MANUAL.
• When the ambient temperature of the device changes too much, such
as when moving from one room at a lower temperature, to another
room at a higher temperature, allow the device to remain in the room
for 30 minutes before using.
• DO NOT use if this device is splashed by or immersed in water.
• High temperature, high pressure, gas sterilizing or immersion
disinfection for this device is not permitted. Refer to the CLEANING &
DISINFECTING section of this INSTRUCTION MANUAL.
• This device may not be suitable for all users. Measurement results
are for reference only. Contact your physician if you have or suspect a
medical condition.
• Data averaging and signal processing have a delay in the upgrade of
SpO2 data values. When the data update period is less than 30 seconds,
the time for obtaining dynamic average values will increase.
• DO NOT use external coloring agents (such as nail polish, articial nails,
or colored skin care products) on the test nger as this may affect the
measurement accuracy.
• Measurement accuracy may be impacted if the test nger:
-Is too cold. Warm the test nger up before using the device.
-Is too thin. Trying using a bigger nger such as the thumb or middle nger.
-Has a long ngernail. Trim the ngernail or switch to a nger with a
short nail.
• Refer to Figure 5 in this INSTRUCTION MANUAL for correct nger
position to use this device. Improper nger position may cause
inaccurate results.
• The light between the photoelectric receiving tube and the light-
emitting tube of this device must pass through the subject’s arteriole.
Make sure the optical path is free from any optical obstacles like
rubberized fabric, to avoid inaccurate results.
• Excessive ambient light may affect the measured results, such
as surgical light (especially xenon light sources), bilirubin lamp,
uorescent lamp, infrared heater and direct sunlight, etc. In order to
prevent interference from ambient light, make sure to place the sensor
properly and cover the sensor with opaque material.
• This device should not be placed on a limb with a blood pressure cuff,
arterial ductus or intraluminal tube.
• The measured value may be inaccurate during debrillation and in
a short period after debrillation, as it does not have a debrillation
function.
• This device has been calibrated before leaving factory.
• This device is calibrated to display functional oxygen saturation.
CLINICAL RESTRICTION
A. As the measurement is taken on the basis of arteriole pulse, substantial
pulsating blood ow of the user is required. For a user with weak pulse
due to shock, low ambient/body temperature, major bleeding, or use
of vascular contracting drug, the SpO2will decrease. In this case, the
measurement will be more sensitive to interference.
B. The measurement will be inuenced by intravascular staining agents (such
as indocyanine green or methylene blue), skin pigmentation.
C. The measured value may be seemingly normal for a user who has anemia
or dysfunctional hemoglobin (such as carboxyhaemoglobin (COHb),
methaemoglobin (MetHb) and sulfhaemoglobin (SuHb), however the user
may appear hypoxia. It is recommended to perform further assessment
according the clinical situation and symptoms.
D. Pulse oxygen only has a reference meaning for anemia and toxic hypoxia,
as some severe anemia patients still show better pulse oxygen measured
values.
E. Contraindication: no
FINGERTIP PULSE OXIMETER COMPONENTS
INSTALLING BATTERIES IN YOUR PULSE OXIMETER
ATTACHING THE LANYARD TO THE PULSE OXIMETER
MEASURING PULSE RATE AND BLOOD OXYGEN LEVELS
Optical sensor
Red light Wavelength: about 660 nm, optical output power: < 6.65 mW
Infrared light Wavelength: about 905 nm, optical output power: < 6.75 mW
Safety class Internally powered equipment, type BF applied part
International
Protection IP22
Working voltage DC 2.6 V - 3.6 V
Working current ≤ 25 mA
Power supply DC 3V (2 x AAA batteries)
Battery life Two batteries can work continually for approximately 24 hours
Dimension 2.2”(L) x 1.2”(W) x 1.3”(H)
57mm(L) x 31mm(W) x 32mm(H)
Weight About 1.8oz /51g (including batteries)
If you have any problems, please do not contact the store.
Contact our customer service at 1-877-383-6399
(8:30 am - 5:00 pm EST) Monday - Friday
or contact us at
Our customer service will be happy to assist you.
© 2021 Sunbeam Products, Inc.All rights reserved.
Distributed by Star Elite Inc., Montreal, Canada H3B 3X9.
SE004-071421
Fingertip Pulse Oximeter Model CMS50DL
Printed in China
Model : CMS50DL
Item: 16979 Figure 3.
Batteries Installation
Power Button
SpO² Display
Battery Level Indicator
Pulse Rate BPM (beats per minute)
Pulse Rate Bar Graph

• Explosive hazard - DO NOT use this device in environment with
inammable gas such as anesthetic.
• DO NOT use this device during an MRI or CT scan, as the induced current
may cause burns.
• DO NOT take the information displayed on this device as the sole basis
for clinical diagnosis. This device is not intended as a replacement for
advice from a physician or medical professional.
• This device is NOT recommended for continuous monitoring.
• DO NOT use on a ngertip that has scar tissue or or that has any other
skin disorder as this may affect the measurement accuracy.
• DO NOT stare directly into the nger chamber of this device while it is
turned on as the infrared light may be damaging to the eyes.
• This device contains silicone, PVC, TPU, TPE and ABS materials, whose
biocompatibility has been tested in accordance with the requirements
in ISO 10993-1, and it has passed the recommended biocompatibility
test. Anyone with allergies to silicone, PVC, TPU, TPE or ABS should not
use this device.
• DO NOT use the included lanyard if you are allergic to nylon/polyester
material.
• DO NOT wrap the lanyard around the neck to avoid an accident.
• This device should be kept out of the reach of children/pets. When
not in use, store this device in a dry room and protect against extreme
moisture, heat, lint, dust and direct sunlight. Never place any heavy
objects on the pulse oximeter.
• DO NOT throw batteries into re.
• DO NOT discard in household trash.
• Only use recommended batteries.
• DO NOT use rechargeable batteries.
• DO NOT drop, disassemble or modify this device.
• DO NOT use this device if you think it is damaged or notice anything
unusual.
• This device comprises sensitive components and must be treated with
caution. Observe the cleaning, maintenance and operating conditions
described in this INSTRUCTION MANUAL.
• DO NOT perform service or maintenance while this device is in use.
• DO NOT touch the battery and the person at the same time.
• DO NOT use this device if it is damaged, degraded or loosened in any way.
• Continuous use of a damaged device may cause injury, improper
results, or serious danger.
• This device needs to be used and serviced in accordance with the
information provided in this INSTRUCTION MANUAL.
OVERVIEW
The oxygen saturation is the percentage of HbO2in the total Hb in the blood,
so-called the O2concentration in the blood, it is an important physiological
parameter for the respiratory and circulatory system.A number of diseases
related to the respiratory system may cause a decrease of SpO2in the blood.
APPLIED RANGE
This Pulse Oximeter is a non-invasive device intended for the spot-check
of arterial hemoglobin (SpO2) and the pulse rate of adults in home use
environments.This device is not intended for continuous monitoring. Solely for
use with sporting and aviation activities. Intended to monitor heart rate
during exercise.
ENVIRONMENTAL REQUIREMENTS
• Storage Environment
a) Temperature: -40°F - 140°F (-40°C - 60°C)
b) Relative humidity: ≤ 95%
c) Atmospheric pressure: 500 hPa - 1060 hPa
• Operation Environment
a) Temperature: 50°F - 104°F (10°C - 40°C)
b) Relative Humidity: ≤ 75%
c) Atmospheric pressure: 700 hPa - 1060 hPa
NOTE: BEFORE YOU BEGIN, WARM THE HANDS AND FINGERS BY
RUBBING THEM TOGETHER.
• Low blood perfusion can affect oximeter readings.
• 99% of reading errors are caused by cold extremities.
• Keep hand and nger still as movement may affect the reading.
• Allow at least 30 seconds for the measurement to stabilize.
• Nail polish and articial ngernails may cause false or no results.
Principle of this Pulse Oximeter is as follows: An experience formula of
data process is established taking use of Lambert Beer Law according to
Spectrum Absorption Characteristics of Reductive Hemoglobin (Hb) and
Oxyhemoglobin (Hb02) in glow & near-infrared zones. Operation principle of
the device is: Photoelectric Oxyhemoglobin Inspection Technology is adopted
in accordance with Capacity Pulse Scanning & Recording Technology, so that
two beams of different wavelength of lights can be focused onto human nail
tip through perspective clamp nger-type sensor. Then measured signal can
be obtained by a photosensitive element, information acquired through
which will be shown on screen through treatment in electronic circuits and
microprocessor.
1. Slide open the back cover and install 2 AAA batteries as shown.To avoid
damage, make sure the batteries align with the “+” and “–” symbols.
2. Replace the back cover.
Figure 4.
Attaching the connector loop
1. Thread the thin connector loop through the hole.
2. Insert the quick-release buckle through the loop.
3. Slide the lanyard clip into the quick-release buckle.
1. Gently squeeze to open the nger chamber.
2. Place nger fully into the chamber.
3. Press the ON button.
Fingertip Pulse Oximeter
Instruction Manual
Figure 1.
Operating principle
AAA
AAA
Figure 2. Fingertip Pulse Oximeter
AAA
AAA
AAA
AAA
Figure 5.
Put nger in position
WARNINGS
PRINCIPLE
TECHNICAL DATA
READ AND SAVE THESE INSTRUCTIONS. PLEASE READ THIS
INSTRUCTION MANUAL BEFORE USING THIS DEVICE. IF THE
USAGE IS NOT FULLY UNDERSTOOD, PLEASE DO NOT USE THIS
PULSE OXIMETER.
ATTENTION
• Use and store this device according the ENVIRONMENTAL
REQUIREMENTS section of this INSTRUCTION MANUAL.
• When the ambient temperature of the device changes too much, such
as when moving from one room at a lower temperature, to another
room at a higher temperature, allow the device to remain in the room
for 30 minutes before using.
• DO NOT use if this device is splashed by or immersed in water.
• High temperature, high pressure, gas sterilizing or immersion
disinfection for this device is not permitted. Refer to the CLEANING &
DISINFECTING section of this INSTRUCTION MANUAL.
• This device may not be suitable for all users. Measurement results
are for reference only. Contact your physician if you have or suspect a
medical condition.
• Data averaging and signal processing have a delay in the upgrade of
SpO2 data values. When the data update period is less than 30 seconds,
the time for obtaining dynamic average values will increase.
• DO NOT use external coloring agents (such as nail polish, articial nails,
or colored skin care products) on the test nger as this may affect the
measurement accuracy.
• Measurement accuracy may be impacted if the test nger:
-Is too cold. Warm the test nger up before using the device.
-Is too thin. Trying using a bigger nger such as the thumb or middle nger.
-Has a long ngernail. Trim the ngernail or switch to a nger with a
short nail.
• Refer to Figure 5 in this INSTRUCTION MANUAL for correct nger
position to use this device. Improper nger position may cause
inaccurate results.
• The light between the photoelectric receiving tube and the light-
emitting tube of this device must pass through the subject’s arteriole.
Make sure the optical path is free from any optical obstacles like
rubberized fabric, to avoid inaccurate results.
• Excessive ambient light may affect the measured results, such
as surgical light (especially xenon light sources), bilirubin lamp,
uorescent lamp, infrared heater and direct sunlight, etc. In order to
prevent interference from ambient light, make sure to place the sensor
properly and cover the sensor with opaque material.
• This device should not be placed on a limb with a blood pressure cuff,
arterial ductus or intraluminal tube.
• The measured value may be inaccurate during debrillation and in
a short period after debrillation, as it does not have a debrillation
function.
• This device has been calibrated before leaving factory.
• This device is calibrated to display functional oxygen saturation.
CLINICAL RESTRICTION
A. As the measurement is taken on the basis of arteriole pulse, substantial
pulsating blood ow of the user is required. For a user with weak pulse
due to shock, low ambient/body temperature, major bleeding, or use
of vascular contracting drug, the SpO2will decrease. In this case, the
measurement will be more sensitive to interference.
B. The measurement will be inuenced by intravascular staining agents (such
as indocyanine green or methylene blue), skin pigmentation.
C. The measured value may be seemingly normal for a user who has anemia
or dysfunctional hemoglobin (such as carboxyhaemoglobin (COHb),
methaemoglobin (MetHb) and sulfhaemoglobin (SuHb), however the user
may appear hypoxia. It is recommended to perform further assessment
according the clinical situation and symptoms.
D. Pulse oxygen only has a reference meaning for anemia and toxic hypoxia,
as some severe anemia patients still show better pulse oxygen measured
values.
E. Contraindication: no
FINGERTIP PULSE OXIMETER COMPONENTS
INSTALLING BATTERIES IN YOUR PULSE OXIMETER
ATTACHING THE LANYARD TO THE PULSE OXIMETER
MEASURING PULSE RATE AND BLOOD OXYGEN LEVELS
Optical sensor
Red light Wavelength: about 660 nm, optical output power: < 6.65 mW
Infrared light Wavelength: about 905 nm, optical output power: < 6.75 mW
Safety class Internally powered equipment, type BF applied part
International
Protection IP22
Working voltage DC 2.6 V - 3.6 V
Working current ≤ 25 mA
Power supply DC 3V (2 x AAA batteries)
Battery life Two batteries can work continually for approximately 24 hours
Dimension 2.2”(L) x 1.2”(W) x 1.3”(H)
57mm(L) x 31mm(W) x 32mm(H)
Weight About 1.8oz /51g (including batteries)
If you have any problems, please do not contact the store.
Contact our customer service at 1-877-383-6399
(8:30 am - 5:00 pm EST) Monday - Friday
or contact us at
Our customer service will be happy to assist you.
© 2021 Sunbeam Products, Inc.All rights reserved.
Distributed by Star Elite Inc., Montreal, Canada H3B 3X9.
SE004-071421
Fingertip Pulse Oximeter Model CMS50DL
Printed in China
Model : CMS50DL
Item: 16979 Figure 3.
Batteries Installation
Power Button
SpO² Display
Battery Level Indicator
Pulse Rate BPM (beats per minute)
Pulse Rate Bar Graph

• Explosive hazard - DO NOT use this device in environment with
inammable gas such as anesthetic.
• DO NOT use this device during an MRI or CT scan, as the induced current
may cause burns.
• DO NOT take the information displayed on this device as the sole basis
for clinical diagnosis. This device is not intended as a replacement for
advice from a physician or medical professional.
• This device is NOT recommended for continuous monitoring.
• DO NOT use on a ngertip that has scar tissue or or that has any other
skin disorder as this may affect the measurement accuracy.
• DO NOT stare directly into the nger chamber of this device while it is
turned on as the infrared light may be damaging to the eyes.
• This device contains silicone, PVC, TPU, TPE and ABS materials, whose
biocompatibility has been tested in accordance with the requirements
in ISO 10993-1, and it has passed the recommended biocompatibility
test. Anyone with allergies to silicone, PVC, TPU, TPE or ABS should not
use this device.
• DO NOT use the included lanyard if you are allergic to nylon/polyester
material.
• DO NOT wrap the lanyard around the neck to avoid an accident.
• This device should be kept out of the reach of children/pets. When
not in use, store this device in a dry room and protect against extreme
moisture, heat, lint, dust and direct sunlight. Never place any heavy
objects on the pulse oximeter.
• DO NOT throw batteries into re.
• DO NOT discard in household trash.
• Only use recommended batteries.
• DO NOT use rechargeable batteries.
• DO NOT drop, disassemble or modify this device.
• DO NOT use this device if you think it is damaged or notice anything
unusual.
• This device comprises sensitive components and must be treated with
caution. Observe the cleaning, maintenance and operating conditions
described in this INSTRUCTION MANUAL.
• DO NOT perform service or maintenance while this device is in use.
• DO NOT touch the battery and the person at the same time.
• DO NOT use this device if it is damaged, degraded or loosened in any way.
• Continuous use of a damaged device may cause injury, improper
results, or serious danger.
• This device needs to be used and serviced in accordance with the
information provided in this INSTRUCTION MANUAL.
OVERVIEW
The oxygen saturation is the percentage of HbO2in the total Hb in the blood,
so-called the O2concentration in the blood, it is an important physiological
parameter for the respiratory and circulatory system.A number of diseases
related to the respiratory system may cause a decrease of SpO2in the blood.
APPLIED RANGE
This Pulse Oximeter is a non-invasive device intended for the spot-check
of arterial hemoglobin (SpO2) and the pulse rate of adults in home use
environments.This device is not intended for continuous monitoring. Solely for
use with sporting and aviation activities. Intended to monitor heart rate
during exercise.
ENVIRONMENTAL REQUIREMENTS
• Storage Environment
a) Temperature: -40°F - 140°F (-40°C - 60°C)
b) Relative humidity: ≤ 95%
c) Atmospheric pressure: 500 hPa - 1060 hPa
• Operation Environment
a) Temperature: 50°F - 104°F (10°C - 40°C)
b) Relative Humidity: ≤ 75%
c) Atmospheric pressure: 700 hPa - 1060 hPa
NOTE: BEFORE YOU BEGIN, WARM THE HANDS AND FINGERS BY
RUBBING THEM TOGETHER.
• Low blood perfusion can affect oximeter readings.
• 99% of reading errors are caused by cold extremities.
• Keep hand and nger still as movement may affect the reading.
• Allow at least 30 seconds for the measurement to stabilize.
• Nail polish and articial ngernails may cause false or no results.
Principle of this Pulse Oximeter is as follows: An experience formula of
data process is established taking use of Lambert Beer Law according to
Spectrum Absorption Characteristics of Reductive Hemoglobin (Hb) and
Oxyhemoglobin (Hb02) in glow & near-infrared zones. Operation principle of
the device is: Photoelectric Oxyhemoglobin Inspection Technology is adopted
in accordance with Capacity Pulse Scanning & Recording Technology, so that
two beams of different wavelength of lights can be focused onto human nail
tip through perspective clamp nger-type sensor. Then measured signal can
be obtained by a photosensitive element, information acquired through
which will be shown on screen through treatment in electronic circuits and
microprocessor.
1. Slide open the back cover and install 2 AAA batteries as shown.To avoid
damage, make sure the batteries align with the “+” and “–” symbols.
2. Replace the back cover.
Figure 4.
Attaching the connector loop
1. Thread the thin connector loop through the hole.
2. Insert the quick-release buckle through the loop.
3. Slide the lanyard clip into the quick-release buckle.
1. Gently squeeze to open the nger chamber.
2. Place nger fully into the chamber.
3. Press the ON button.
Fingertip Pulse Oximeter
Instruction Manual
Figure 1.
Operating principle
AAA
AAA
Figure 2. Fingertip Pulse Oximeter
AAA
AAA
AAA
AAA
Figure 5.
Put nger in position
WARNINGS
PRINCIPLE
TECHNICAL DATA
READ AND SAVE THESE INSTRUCTIONS. PLEASE READ THIS
INSTRUCTION MANUAL BEFORE USING THIS DEVICE. IF THE
USAGE IS NOT FULLY UNDERSTOOD, PLEASE DO NOT USE THIS
PULSE OXIMETER.
ATTENTION
• Use and store this device according the ENVIRONMENTAL
REQUIREMENTS section of this INSTRUCTION MANUAL.
• When the ambient temperature of the device changes too much, such
as when moving from one room at a lower temperature, to another
room at a higher temperature, allow the device to remain in the room
for 30 minutes before using.
• DO NOT use if this device is splashed by or immersed in water.
• High temperature, high pressure, gas sterilizing or immersion
disinfection for this device is not permitted. Refer to the CLEANING &
DISINFECTING section of this INSTRUCTION MANUAL.
• This device may not be suitable for all users. Measurement results
are for reference only. Contact your physician if you have or suspect a
medical condition.
• Data averaging and signal processing have a delay in the upgrade of
SpO2 data values. When the data update period is less than 30 seconds,
the time for obtaining dynamic average values will increase.
• DO NOT use external coloring agents (such as nail polish, articial nails,
or colored skin care products) on the test nger as this may affect the
measurement accuracy.
• Measurement accuracy may be impacted if the test nger:
-Is too cold. Warm the test nger up before using the device.
-Is too thin. Trying using a bigger nger such as the thumb or middle nger.
-Has a long ngernail. Trim the ngernail or switch to a nger with a
short nail.
• Refer to Figure 5 in this INSTRUCTION MANUAL for correct nger
position to use this device. Improper nger position may cause
inaccurate results.
• The light between the photoelectric receiving tube and the light-
emitting tube of this device must pass through the subject’s arteriole.
Make sure the optical path is free from any optical obstacles like
rubberized fabric, to avoid inaccurate results.
• Excessive ambient light may affect the measured results, such
as surgical light (especially xenon light sources), bilirubin lamp,
uorescent lamp, infrared heater and direct sunlight, etc. In order to
prevent interference from ambient light, make sure to place the sensor
properly and cover the sensor with opaque material.
• This device should not be placed on a limb with a blood pressure cuff,
arterial ductus or intraluminal tube.
• The measured value may be inaccurate during debrillation and in
a short period after debrillation, as it does not have a debrillation
function.
• This device has been calibrated before leaving factory.
• This device is calibrated to display functional oxygen saturation.
CLINICAL RESTRICTION
A. As the measurement is taken on the basis of arteriole pulse, substantial
pulsating blood ow of the user is required. For a user with weak pulse
due to shock, low ambient/body temperature, major bleeding, or use
of vascular contracting drug, the SpO2will decrease. In this case, the
measurement will be more sensitive to interference.
B. The measurement will be inuenced by intravascular staining agents (such
as indocyanine green or methylene blue), skin pigmentation.
C. The measured value may be seemingly normal for a user who has anemia
or dysfunctional hemoglobin (such as carboxyhaemoglobin (COHb),
methaemoglobin (MetHb) and sulfhaemoglobin (SuHb), however the user
may appear hypoxia. It is recommended to perform further assessment
according the clinical situation and symptoms.
D. Pulse oxygen only has a reference meaning for anemia and toxic hypoxia,
as some severe anemia patients still show better pulse oxygen measured
values.
E. Contraindication: no
FINGERTIP PULSE OXIMETER COMPONENTS
INSTALLING BATTERIES IN YOUR PULSE OXIMETER
ATTACHING THE LANYARD TO THE PULSE OXIMETER
MEASURING PULSE RATE AND BLOOD OXYGEN LEVELS
Optical sensor
Red light Wavelength: about 660 nm, optical output power: < 6.65 mW
Infrared light Wavelength: about 905 nm, optical output power: < 6.75 mW
Safety class Internally powered equipment, type BF applied part
International
Protection IP22
Working voltage DC 2.6 V - 3.6 V
Working current ≤ 25 mA
Power supply DC 3V (2 x AAA batteries)
Battery life Two batteries can work continually for approximately 24 hours
Dimension 2.2”(L) x 1.2”(W) x 1.3”(H)
57mm(L) x 31mm(W) x 32mm(H)
Weight About 1.8oz /51g (including batteries)
If you have any problems, please do not contact the store.
Contact our customer service at 1-877-383-6399
(8:30 am - 5:00 pm EST) Monday - Friday
or contact us at
Our customer service will be happy to assist you.
© 2021 Sunbeam Products, Inc.All rights reserved.
Distributed by Star Elite Inc., Montreal, Canada H3B 3X9.
SE004-071421
Fingertip Pulse Oximeter Model CMS50DL
Printed in China
Model : CMS50DL
Item: 16979 Figure 3.
Batteries Installation
Power Button
SpO² Display
Battery Level Indicator
Pulse Rate BPM (beats per minute)
Pulse Rate Bar Graph

NOTES
• At 80 MHz and 800 MHz, the higher frequency range applies.
• These guidelines may not apply in all situations. Electromagnetic
propagation is affected by absorption and reection from structures,
objects and people.
a Field strengths from xed transmitters, such as base stations for radio
(cellular/cordless) telephones and land mobile radios, amateur radio, AM
and FM radio broadcast and TV broadcast cannot be predicted theoretically
with accuracy. To assess the electromagnetic environment due to xed
RF transmitters, an electromagnetic site survey should be considered. If
the measured eld strength in the location in which this Pulse Oximeter
is used exceeds the applicable RF compliance level above, this Pulse
Oximeter should be observed to verify normal operation. If abnormal
performance is observed, additional measures may be necessary, such as
reorienting or relocating this Pulse Oximeter.
b Over the frequency range 150 kHz to 80 MHz, eld strengths should be
less than 3 V/M.
Recommended separation distances between
portable and mobile RF communications equipment and the
Pulse Oximeter.
This Pulse Oximeter is intended for use in an electromagnetic environment
in which radiated RF disturbances are controlled.The user of this Pulse
Oximeter can help prevent electromagnetic interference by maintaining
a minimum distance between portable and mobile RF communications
equipment (transmitters) and this Pulse Oximeter as recommended
below, according to the maximum output power of the communications
equipment.
Rated
maximum
output
power of
transmitter
(W)
Separation distance according to frequency of
transmitter(m)
150 kHz to 80 MHz 80 MHz to 800 MHz 800 MHz to 2.7 GHz
0.01 0.058 0.035 0.07
0.1 0.18 0.11 0.22
10.58 0.35 0.7
10 1.83 1.10 2.21
100 5.8 3.5 7
For transmitters rated at a maximum output power not listed above, the
recommended separation distance d in metres (m) can be estimated using
the equation applicable to the frequency of the transmitter, where P is the
maximum output power rating of the transmitter in watts (W) according to
the transmitter manufacturer.
NOTES:
• At 80 MHz and 800 MHz, the separation distance for the higher
frequency range applies.
• These guidelines may not apply in all situations. Electromagnetic
propagation is affected by absorption and reection from structures,
objects and people.
GUIDANCE AND MANUFACTURER’S DECLARATION –
ELECTROMAGNETIC EMISSIONS –
FOR ALL EQUIPMENT AND SYSTEMS
GUIDANCE AND MANUFACTURER’S DECLARATION –
ELECTROMAGNETIC IMMUNITY –
FOR ALL EQUIPMENT AND SYSTEMS
RECOMMENDED SEPARATION DISTANCES BETWEEN
PORTABLE AND MOBILE
RF COMMUNICATIONS EQUIPMENT AND THE EQUIP-
MENT OR SYSTEM – FOR EQUIPMENT OR SYSTEM THAT
ARE NOT LIFE-SUPPORTING
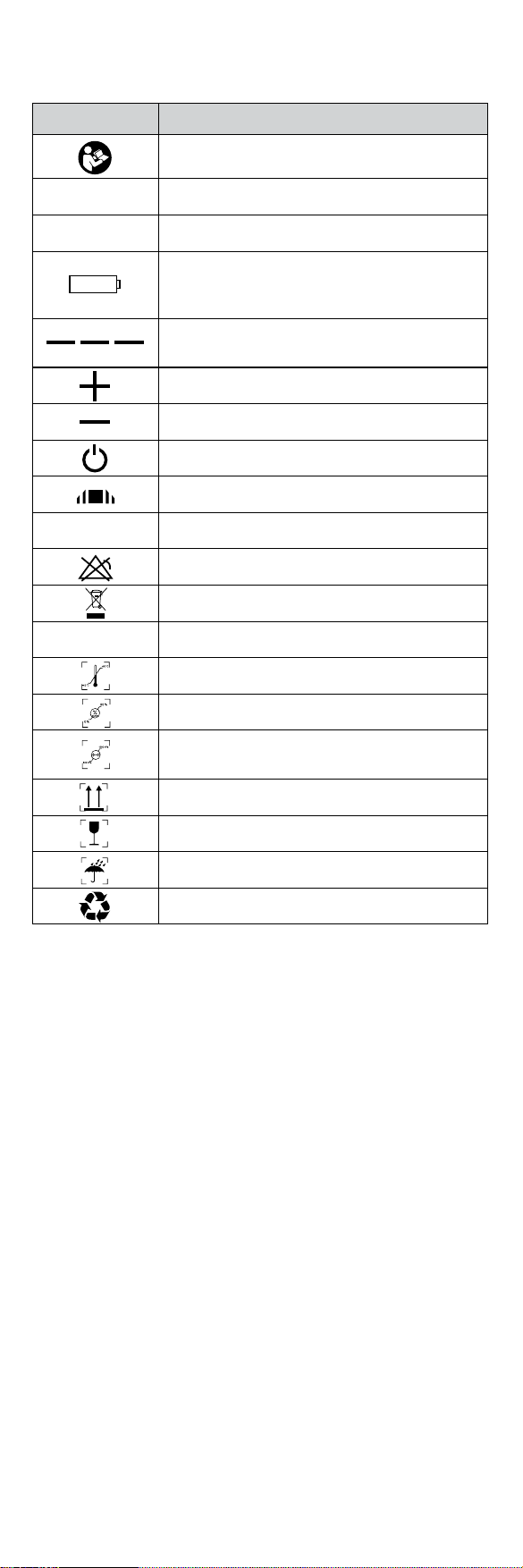
Symbol Description
Refer to instruction manual/booklet
%SpO2Pulse oxygen saturation (%)
PRbpm Pulse rate (beats/minute)
The battery voltage is decient
(change the battery to avoid inaccurate
measurements)
1) No nger inserted
2) Inadequate signal
Battery positive electrode
Battery cathode
Exit standby mode
Adjust screen brightness
SN Serial number
Alarm inhibit
WEEE (2002/96/EC)
IP22 Ingress of liquids rank
Storage and Transport Temperature limitation
Storage and Transport Humidity limitation
Storage and Transport Atmospheric pressure
limitation
This side up
Fragile, handle with care
Keep dry
Recyclable
EMC DECLARATION
• The device is subject to special EMC precautions and it must be installed
and used in accordance with these guidelines.
• The electromagnetic eld can affect the device performance, so other
equipment used near the device must meet the corresponding EMC
requirements. Mobile phones, X-rays or MRI devices are possible
interference source, as they can emit high-intensity electromagnetic
radiation.
• Refer to above chapters for the minimum value of user’s physiological
signal. Inaccurate result will appear when the device operates with the
values lower than the descriptions in above chapter.
• The use of ACCESSORIES, transducers and cables other than those
specied, with the exception of transducers and cables sold by the
MANUFACTURER of the ME EQUIPMENT or ME SYSTEM as replacement
parts for internal components, may result in increased EMISSIONS or
decreased IMMUNITY of the ME EQUIPMENT or ME SYSTEM.
• This device should not be used adjacent to or stacked with other
equipment and that if adjacent or stacked use is necessary, it should be
observed to verify normal operation in the conguration in which it will
be used.
• Devices or systems may still be interfered by other equipment, even if
other equipment meets the requirements of the corresponding national
standard.
• Basic performance: SpO2 measured range: 70% - 100%, absolute error:
±2%; Pulse Rate measured range: 30 - 250 beats/minute, accuracy: ±2
beats/minute or ±2%, whichever is greater.
Guidance and manufacturer’s declaration – electromagnetic emission
This Pulse Oximeter is intended for use in the electromagnetic environment speci-
ed below. The user of this Pulse Oximeter should assure that it is used in such and
environment.
Emission test Compliance Electromagnetic
environment – guidance
RF emissions
CISPR 11 Group 1
This Pulse Oximeter uses RF energy only for its
internal function. Therefore, its RF emissions are very
low and are not likely to cause any interference in
nearby electronic equipment.
RF emissions
CISPR 11 Class B
This Pulse Oximeter is suitable for use in all
establishments, including domestic establishments
and those directly connected to the public
low-voltage power supply network that supplies
buildings used for domestic purposes.
Guidance and manufacturer’s declaration – electromagnetic immunity
This Pulse Oximeter is intended for use in the electromagnetic environment speci-
ed below. The user of this Pulse Oximeter should assure that it is used in such an
environment.
Immunity
test
IEC 60601
test level
Compliance
level
Electromagnetic environment -
guidance
Electrostatic
discharge (ESD)
IEC 61000-4-2
8 kV contact
15 kV air
8 kV contact
15 kV air
Floors should be wood, concrete or
ceramic tile. If the oor is covered
with synthetic material, the relative
humidity should be at least 30%. the
manufacturer may recommend the
ESD precautionary procedures to user.
Power frequency
(50Hz) magnetic
eld
IEC 61000-4-8
30A/m 30A/m
Power frequency magnetic elds
should be at levels characteristic
of a typical location in a typical
commercial or hospital environment.
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
3V (0.15MHz–
80MHz), 6V
(in ISM bands
between
0.15MHz and
80MHz)
10 V/m
80 MHz to
2.7GH
3V
(0.15MHz–
80MHz),
6V (in ISM
bands
between
0.15MHz
and 80MHz)
10 V/m
Portable and mobile RF
communications equipment
should be used no closer to
any part of the Pulse Oximeter,
including cables, than the
recommended separation
distance calculated from the
equation applicable to the
frequency of the transmitter.
Recommended separation
distance
80 MHz to 800 MHz
800 MHz to 2.7 GHz
Where P is the maximum output
power rating of the transmitter
in watts (W) according to the
transmitter manufacturer and d
is the recommended separation
distance in metres (m).
Field strengths from xed RF
transmitters, as determined
by an electromagnetic site
survey,ashould be less than
the compliance level in each
frequency range.b
Interference may occur in the
vicinity of equipment marked
with the following symbol:
KEY OF SYMBOLS
Note: This device and INSTRUCTION MANUAL may not contain all the following
symbols.
READING THE RESULTS
SpO2
Display range 0% - 99%
Measured range 0% - 100%
Accuracy 70% - 100%: ±2%;
0% - 69%: unspecied.
Resolution 1%
Pulse Rate (PR)
Display range 30 - 250 beats/minute
Measured range 30 - 250 beats/minute
Accuracy ±2 beats/minute or ±2%, whichever is greater
Resolution 1 beat/minute
Accuracy under low
perfusion
Low perfusion 0.4%:
SpO2: ±4%;
Pulse Rate: ±2 beats/minute
or ±2%, whichever is greater
Light interference Under normal and ambient light conditions,
the SpO2 deviation ≤ 1%
CLEANING & DISINFECTING THE PULSE OXIMETER
1. Remove batteries before cleaning.
2. Wipe nger chamber and exterior surface with a disinfecting solution of at
least 75% isopropyl alcohol.
• Do not immerse in water or liquid.
• Do not spray any liquid directly onto the device.
3. Allow to air-dry or wipe dry with a clean, soft cloth.
HIGH-PRESSURE STERILIZATION CANNOT BE USED ON THE DEVICE
MAINTAINING & STORING THE PULSE OXIMETER
1. Inspect this device regularly. Discontinue use if you notice visible damage.
2. Replace the batteries when low-battery indicator appears or device does
not turn on.
3.Remove the batteries before storage or when not in use for an extended
timeframe.
4. Make sure the device is clean and dry before storage.
5. Store this device in a dry, well-ventilated location away from extreme
temperatures.
DISPOSAL OF DEVICE & BATTERIES
At the end of its useful product life, dispose of/recycle this device and used
batteries following local regulations to avoid environmental pollution.
• DO NOT discard in household trash.
• NEVER dispose of batteries in re.
• Dispose of/recycle used batteries following local regulations to avoid
environmental pollution.
LIMITED 2-YEAR WARRANTY
This product* has a limited warranty of 2 years from the original date of
purchase against workmanship and defects in material. If under normal use,
your product fails to operate, please contact our customer service department
with proof of purchase. Star Elite Inc. may deny claims of damage caused by
misuse or modications of this product.
*Excluding accessories (Batteries, Lanyard & Carrying case)
FAQS / TROUBLESHOOTING
Q: The pulse oximeter does not turn on.
A: Check the batteries for correct installation. Replace both AAA batteries
when the battery indicator is low.
Q: The display screen turns off suddenly.
A: The device enters energy-mode when not in use for several seconds. Press
ON button.The batteries are low. Install 2 new AAA batteries.
Q: The display is not showing SP02and Pulse Rate.
A: Make sure the nger is fully inserted into the nger chamber. Remain still
while measuring. Nail polish or articial nails may interfere with an
accurate reading.
NOTES
• At 80 MHz and 800 MHz, the higher frequency range applies.
• These guidelines may not apply in all situations. Electromagnetic
propagation is affected by absorption and reection from structures,
objects and people.
a Field strengths from xed transmitters, such as base stations for radio
(cellular/cordless) telephones and land mobile radios, amateur radio, AM
and FM radio broadcast and TV broadcast cannot be predicted theoretically
with accuracy. To assess the electromagnetic environment due to xed
RF transmitters, an electromagnetic site survey should be considered. If
the measured eld strength in the location in which this Pulse Oximeter
is used exceeds the applicable RF compliance level above, this Pulse
Oximeter should be observed to verify normal operation. If abnormal
performance is observed, additional measures may be necessary, such as
reorienting or relocating this Pulse Oximeter.
b Over the frequency range 150 kHz to 80 MHz, eld strengths should be
less than 3 V/M.
Recommended separation distances between
portable and mobile RF communications equipment and the
Pulse Oximeter.
This Pulse Oximeter is intended for use in an electromagnetic environment
in which radiated RF disturbances are controlled.The user of this Pulse
Oximeter can help prevent electromagnetic interference by maintaining
a minimum distance between portable and mobile RF communications
equipment (transmitters) and this Pulse Oximeter as recommended
below, according to the maximum output power of the communications
equipment.
Rated
maximum
output
power of
transmitter
(W)
Separation distance according to frequency of
transmitter(m)
150 kHz to 80 MHz 80 MHz to 800 MHz 800 MHz to 2.7 GHz
0.01 0.058 0.035 0.07
0.1 0.18 0.11 0.22
10.58 0.35 0.7
10 1.83 1.10 2.21
100 5.8 3.5 7
For transmitters rated at a maximum output power not listed above, the
recommended separation distance d in metres (m) can be estimated using
the equation applicable to the frequency of the transmitter, where P is the
maximum output power rating of the transmitter in watts (W) according to
the transmitter manufacturer.
NOTES:
• At 80 MHz and 800 MHz, the separation distance for the higher
frequency range applies.
• These guidelines may not apply in all situations. Electromagnetic
propagation is affected by absorption and reection from structures,
objects and people.
GUIDANCE AND MANUFACTURER’S DECLARATION –
ELECTROMAGNETIC EMISSIONS –
FOR ALL EQUIPMENT AND SYSTEMS
GUIDANCE AND MANUFACTURER’S DECLARATION –
ELECTROMAGNETIC IMMUNITY –
FOR ALL EQUIPMENT AND SYSTEMS
RECOMMENDED SEPARATION DISTANCES BETWEEN
PORTABLE AND MOBILE
RF COMMUNICATIONS EQUIPMENT AND THE EQUIP-
MENT OR SYSTEM – FOR EQUIPMENT OR SYSTEM THAT
ARE NOT LIFE-SUPPORTING
Symbol Description
Refer to instruction manual/booklet
%SpO2Pulse oxygen saturation (%)
PRbpm Pulse rate (beats/minute)
The battery voltage is decient
(change the battery to avoid inaccurate
measurements)
1) No nger inserted
2) Inadequate signal
Battery positive electrode
Battery cathode
Exit standby mode
Adjust screen brightness
SN Serial number
Alarm inhibit
WEEE (2002/96/EC)
IP22 Ingress of liquids rank
Storage and Transport Temperature limitation
Storage and Transport Humidity limitation
Storage and Transport Atmospheric pressure
limitation
This side up
Fragile, handle with care
Keep dry
Recyclable
EMC DECLARATION
• The device is subject to special EMC precautions and it must be installed
and used in accordance with these guidelines.
• The electromagnetic eld can affect the device performance, so other
equipment used near the device must meet the corresponding EMC
requirements. Mobile phones, X-rays or MRI devices are possible
interference source, as they can emit high-intensity electromagnetic
radiation.
• Refer to above chapters for the minimum value of user’s physiological
signal. Inaccurate result will appear when the device operates with the
values lower than the descriptions in above chapter.
• The use of ACCESSORIES, transducers and cables other than those
specied, with the exception of transducers and cables sold by the
MANUFACTURER of the ME EQUIPMENT or ME SYSTEM as replacement
parts for internal components, may result in increased EMISSIONS or
decreased IMMUNITY of the ME EQUIPMENT or ME SYSTEM.
• This device should not be used adjacent to or stacked with other
equipment and that if adjacent or stacked use is necessary, it should be
observed to verify normal operation in the conguration in which it will
be used.
• Devices or systems may still be interfered by other equipment, even if
other equipment meets the requirements of the corresponding national
standard.
• Basic performance: SpO2 measured range: 70% - 100%, absolute error:
±2%; Pulse Rate measured range: 30 - 250 beats/minute, accuracy: ±2
beats/minute or ±2%, whichever is greater.
Guidance and manufacturer’s declaration – electromagnetic emission
This Pulse Oximeter is intended for use in the electromagnetic environment speci-
ed below. The user of this Pulse Oximeter should assure that it is used in such and
environment.
Emission test Compliance Electromagnetic
environment – guidance
RF emissions
CISPR 11 Group 1
This Pulse Oximeter uses RF energy only for its
internal function. Therefore, its RF emissions are very
low and are not likely to cause any interference in
nearby electronic equipment.
RF emissions
CISPR 11 Class B
This Pulse Oximeter is suitable for use in all
establishments, including domestic establishments
and those directly connected to the public
low-voltage power supply network that supplies
buildings used for domestic purposes.
Guidance and manufacturer’s declaration – electromagnetic immunity
This Pulse Oximeter is intended for use in the electromagnetic environment speci-
ed below. The user of this Pulse Oximeter should assure that it is used in such an
environment.
Immunity
test
IEC 60601
test level
Compliance
level
Electromagnetic environment -
guidance
Electrostatic
discharge (ESD)
IEC 61000-4-2
8 kV contact
15 kV air
8 kV contact
15 kV air
Floors should be wood, concrete or
ceramic tile. If the oor is covered
with synthetic material, the relative
humidity should be at least 30%. the
manufacturer may recommend the
ESD precautionary procedures to user.
Power frequency
(50Hz) magnetic
eld
IEC 61000-4-8
30A/m 30A/m
Power frequency magnetic elds
should be at levels characteristic
of a typical location in a typical
commercial or hospital environment.
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
3V (0.15MHz–
80MHz), 6V
(in ISM bands
between
0.15MHz and
80MHz)
10 V/m
80 MHz to
2.7GH
3V
(0.15MHz–
80MHz),
6V (in ISM
bands
between
0.15MHz
and 80MHz)
10 V/m
Portable and mobile RF
communications equipment
should be used no closer to
any part of the Pulse Oximeter,
including cables, than the
recommended separation
distance calculated from the
equation applicable to the
frequency of the transmitter.
Recommended separation
distance
80 MHz to 800 MHz
800 MHz to 2.7 GHz
Where P is the maximum output
power rating of the transmitter
in watts (W) according to the
transmitter manufacturer and d
is the recommended separation
distance in metres (m).
Field strengths from xed RF
transmitters, as determined
by an electromagnetic site
survey,ashould be less than
the compliance level in each
frequency range.b
Interference may occur in the
vicinity of equipment marked
with the following symbol:
KEY OF SYMBOLS
Note: This device and INSTRUCTION MANUAL may not contain all the following
symbols.
READING THE RESULTS
SpO2
Display range 0% - 99%
Measured range 0% - 100%
Accuracy 70% - 100%: ±2%;
0% - 69%: unspecied.
Resolution 1%
Pulse Rate (PR)
Display range 30 - 250 beats/minute
Measured range 30 - 250 beats/minute
Accuracy ±2 beats/minute or ±2%, whichever is greater
Resolution 1 beat/minute
Accuracy under low
perfusion
Low perfusion 0.4%:
SpO2: ±4%;
Pulse Rate: ±2 beats/minute
or ±2%, whichever is greater
Light interference Under normal and ambient light conditions,
the SpO2 deviation ≤ 1%
CLEANING & DISINFECTING THE PULSE OXIMETER
1. Remove batteries before cleaning.
2. Wipe nger chamber and exterior surface with a disinfecting solution of at
least 75% isopropyl alcohol.
• Do not immerse in water or liquid.
• Do not spray any liquid directly onto the device.
3. Allow to air-dry or wipe dry with a clean, soft cloth.
HIGH-PRESSURE STERILIZATION CANNOT BE USED ON THE DEVICE
MAINTAINING & STORING THE PULSE OXIMETER
1. Inspect this device regularly. Discontinue use if you notice visible damage.
2. Replace the batteries when low-battery indicator appears or device does
not turn on.
3.Remove the batteries before storage or when not in use for an extended
timeframe.
4. Make sure the device is clean and dry before storage.
5. Store this device in a dry, well-ventilated location away from extreme
temperatures.
DISPOSAL OF DEVICE & BATTERIES
At the end of its useful product life, dispose of/recycle this device and used
batteries following local regulations to avoid environmental pollution.
• DO NOT discard in household trash.
• NEVER dispose of batteries in re.
• Dispose of/recycle used batteries following local regulations to avoid
environmental pollution.
LIMITED 2-YEAR WARRANTY
This product* has a limited warranty of 2 years from the original date of
purchase against workmanship and defects in material. If under normal use,
your product fails to operate, please contact our customer service department
with proof of purchase. Star Elite Inc. may deny claims of damage caused by
misuse or modications of this product.
*Excluding accessories (Batteries, Lanyard & Carrying case)
FAQS / TROUBLESHOOTING
Q: The pulse oximeter does not turn on.
A: Check the batteries for correct installation. Replace both AAA batteries
when the battery indicator is low.
Q: The display screen turns off suddenly.
A: The device enters energy-mode when not in use for several seconds. Press
ON button.The batteries are low. Install 2 new AAA batteries.
Q: The display is not showing SP02and Pulse Rate.
A: Make sure the nger is fully inserted into the nger chamber. Remain still
while measuring. Nail polish or articial nails may interfere with an
accurate reading.

NOTES
• At 80 MHz and 800 MHz, the higher frequency range applies.
• These guidelines may not apply in all situations. Electromagnetic
propagation is affected by absorption and reection from structures,
objects and people.
a Field strengths from xed transmitters, such as base stations for radio
(cellular/cordless) telephones and land mobile radios, amateur radio, AM
and FM radio broadcast and TV broadcast cannot be predicted theoretically
with accuracy. To assess the electromagnetic environment due to xed
RF transmitters, an electromagnetic site survey should be considered. If
the measured eld strength in the location in which this Pulse Oximeter
is used exceeds the applicable RF compliance level above, this Pulse
Oximeter should be observed to verify normal operation. If abnormal
performance is observed, additional measures may be necessary, such as
reorienting or relocating this Pulse Oximeter.
b Over the frequency range 150 kHz to 80 MHz, eld strengths should be
less than 3 V/M.
Recommended separation distances between
portable and mobile RF communications equipment and the
Pulse Oximeter.
This Pulse Oximeter is intended for use in an electromagnetic environment
in which radiated RF disturbances are controlled.The user of this Pulse
Oximeter can help prevent electromagnetic interference by maintaining
a minimum distance between portable and mobile RF communications
equipment (transmitters) and this Pulse Oximeter as recommended
below, according to the maximum output power of the communications
equipment.
Rated
maximum
output
power of
transmitter
(W)
Separation distance according to frequency of
transmitter(m)
150 kHz to 80 MHz 80 MHz to 800 MHz 800 MHz to 2.7 GHz
0.01 0.058 0.035 0.07
0.1 0.18 0.11 0.22
10.58 0.35 0.7
10 1.83 1.10 2.21
100 5.8 3.5 7
For transmitters rated at a maximum output power not listed above, the
recommended separation distance d in metres (m) can be estimated using
the equation applicable to the frequency of the transmitter, where P is the
maximum output power rating of the transmitter in watts (W) according to
the transmitter manufacturer.
NOTES:
• At 80 MHz and 800 MHz, the separation distance for the higher
frequency range applies.
• These guidelines may not apply in all situations. Electromagnetic
propagation is affected by absorption and reection from structures,
objects and people.
GUIDANCE AND MANUFACTURER’S DECLARATION –
ELECTROMAGNETIC EMISSIONS –
FOR ALL EQUIPMENT AND SYSTEMS
GUIDANCE AND MANUFACTURER’S DECLARATION –
ELECTROMAGNETIC IMMUNITY –
FOR ALL EQUIPMENT AND SYSTEMS
RECOMMENDED SEPARATION DISTANCES BETWEEN
PORTABLE AND MOBILE
RF COMMUNICATIONS EQUIPMENT AND THE EQUIP-
MENT OR SYSTEM – FOR EQUIPMENT OR SYSTEM THAT
ARE NOT LIFE-SUPPORTING
Symbol Description
Refer to instruction manual/booklet
%SpO2Pulse oxygen saturation (%)
PRbpm Pulse rate (beats/minute)
The battery voltage is decient
(change the battery to avoid inaccurate
measurements)
1) No nger inserted
2) Inadequate signal
Battery positive electrode
Battery cathode
Exit standby mode
Adjust screen brightness
SN Serial number
Alarm inhibit
WEEE (2002/96/EC)
IP22 Ingress of liquids rank
Storage and Transport Temperature limitation
Storage and Transport Humidity limitation
Storage and Transport Atmospheric pressure
limitation
This side up
Fragile, handle with care
Keep dry
Recyclable
EMC DECLARATION
• The device is subject to special EMC precautions and it must be installed
and used in accordance with these guidelines.
• The electromagnetic eld can affect the device performance, so other
equipment used near the device must meet the corresponding EMC
requirements. Mobile phones, X-rays or MRI devices are possible
interference source, as they can emit high-intensity electromagnetic
radiation.
• Refer to above chapters for the minimum value of user’s physiological
signal. Inaccurate result will appear when the device operates with the
values lower than the descriptions in above chapter.
• The use of ACCESSORIES, transducers and cables other than those
specied, with the exception of transducers and cables sold by the
MANUFACTURER of the ME EQUIPMENT or ME SYSTEM as replacement
parts for internal components, may result in increased EMISSIONS or
decreased IMMUNITY of the ME EQUIPMENT or ME SYSTEM.
• This device should not be used adjacent to or stacked with other
equipment and that if adjacent or stacked use is necessary, it should be
observed to verify normal operation in the conguration in which it will
be used.
• Devices or systems may still be interfered by other equipment, even if
other equipment meets the requirements of the corresponding national
standard.
• Basic performance: SpO2 measured range: 70% - 100%, absolute error:
±2%; Pulse Rate measured range: 30 - 250 beats/minute, accuracy: ±2
beats/minute or ±2%, whichever is greater.
Guidance and manufacturer’s declaration – electromagnetic emission
This Pulse Oximeter is intended for use in the electromagnetic environment speci-
ed below. The user of this Pulse Oximeter should assure that it is used in such and
environment.
Emission test Compliance Electromagnetic
environment – guidance
RF emissions
CISPR 11 Group 1
This Pulse Oximeter uses RF energy only for its
internal function. Therefore, its RF emissions are very
low and are not likely to cause any interference in
nearby electronic equipment.
RF emissions
CISPR 11 Class B
This Pulse Oximeter is suitable for use in all
establishments, including domestic establishments
and those directly connected to the public
low-voltage power supply network that supplies
buildings used for domestic purposes.
Guidance and manufacturer’s declaration – electromagnetic immunity
This Pulse Oximeter is intended for use in the electromagnetic environment speci-
ed below. The user of this Pulse Oximeter should assure that it is used in such an
environment.
Immunity
test
IEC 60601
test level
Compliance
level
Electromagnetic environment -
guidance
Electrostatic
discharge (ESD)
IEC 61000-4-2
8 kV contact
15 kV air
8 kV contact
15 kV air
Floors should be wood, concrete or
ceramic tile. If the oor is covered
with synthetic material, the relative
humidity should be at least 30%. the
manufacturer may recommend the
ESD precautionary procedures to user.
Power frequency
(50Hz) magnetic
eld
IEC 61000-4-8
30A/m 30A/m
Power frequency magnetic elds
should be at levels characteristic
of a typical location in a typical
commercial or hospital environment.
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
3V (0.15MHz–
80MHz), 6V
(in ISM bands
between
0.15MHz and
80MHz)
10 V/m
80 MHz to
2.7GH
3V
(0.15MHz–
80MHz),
6V (in ISM
bands
between
0.15MHz
and 80MHz)
10 V/m
Portable and mobile RF
communications equipment
should be used no closer to
any part of the Pulse Oximeter,
including cables, than the
recommended separation
distance calculated from the
equation applicable to the
frequency of the transmitter.
Recommended separation
distance
80 MHz to 800 MHz
800 MHz to 2.7 GHz
Where P is the maximum output
power rating of the transmitter
in watts (W) according to the
transmitter manufacturer and d
is the recommended separation
distance in metres (m).
Field strengths from xed RF
transmitters, as determined
by an electromagnetic site
survey,ashould be less than
the compliance level in each
frequency range.b
Interference may occur in the
vicinity of equipment marked
with the following symbol:
KEY OF SYMBOLS
Note: This device and INSTRUCTION MANUAL may not contain all the following
symbols.
READING THE RESULTS
SpO2
Display range 0% - 99%
Measured range 0% - 100%
Accuracy 70% - 100%: ±2%;
0% - 69%: unspecied.
Resolution 1%
Pulse Rate (PR)
Display range 30 - 250 beats/minute
Measured range 30 - 250 beats/minute
Accuracy ±2 beats/minute or ±2%, whichever is greater
Resolution 1 beat/minute
Accuracy under low
perfusion
Low perfusion 0.4%:
SpO2: ±4%;
Pulse Rate: ±2 beats/minute
or ±2%, whichever is greater
Light interference Under normal and ambient light conditions,
the SpO2 deviation ≤ 1%
CLEANING & DISINFECTING THE PULSE OXIMETER
1. Remove batteries before cleaning.
2. Wipe nger chamber and exterior surface with a disinfecting solution of at
least 75% isopropyl alcohol.
• Do not immerse in water or liquid.
• Do not spray any liquid directly onto the device.
3. Allow to air-dry or wipe dry with a clean, soft cloth.
HIGH-PRESSURE STERILIZATION CANNOT BE USED ON THE DEVICE
MAINTAINING & STORING THE PULSE OXIMETER
1. Inspect this device regularly. Discontinue use if you notice visible damage.
2. Replace the batteries when low-battery indicator appears or device does
not turn on.
3.Remove the batteries before storage or when not in use for an extended
timeframe.
4. Make sure the device is clean and dry before storage.
5. Store this device in a dry, well-ventilated location away from extreme
temperatures.
DISPOSAL OF DEVICE & BATTERIES
At the end of its useful product life, dispose of/recycle this device and used
batteries following local regulations to avoid environmental pollution.
• DO NOT discard in household trash.
• NEVER dispose of batteries in re.
• Dispose of/recycle used batteries following local regulations to avoid
environmental pollution.
LIMITED 2-YEAR WARRANTY
This product* has a limited warranty of 2 years from the original date of
purchase against workmanship and defects in material. If under normal use,
your product fails to operate, please contact our customer service department
with proof of purchase. Star Elite Inc. may deny claims of damage caused by
misuse or modications of this product.
*Excluding accessories (Batteries, Lanyard & Carrying case)
FAQS / TROUBLESHOOTING
Q: The pulse oximeter does not turn on.
A: Check the batteries for correct installation. Replace both AAA batteries
when the battery indicator is low.
Q: The display screen turns off suddenly.
A: The device enters energy-mode when not in use for several seconds. Press
ON button.The batteries are low. Install 2 new AAA batteries.
Q: The display is not showing SP02and Pulse Rate.
A: Make sure the nger is fully inserted into the nger chamber. Remain still
while measuring. Nail polish or articial nails may interfere with an
accurate reading.
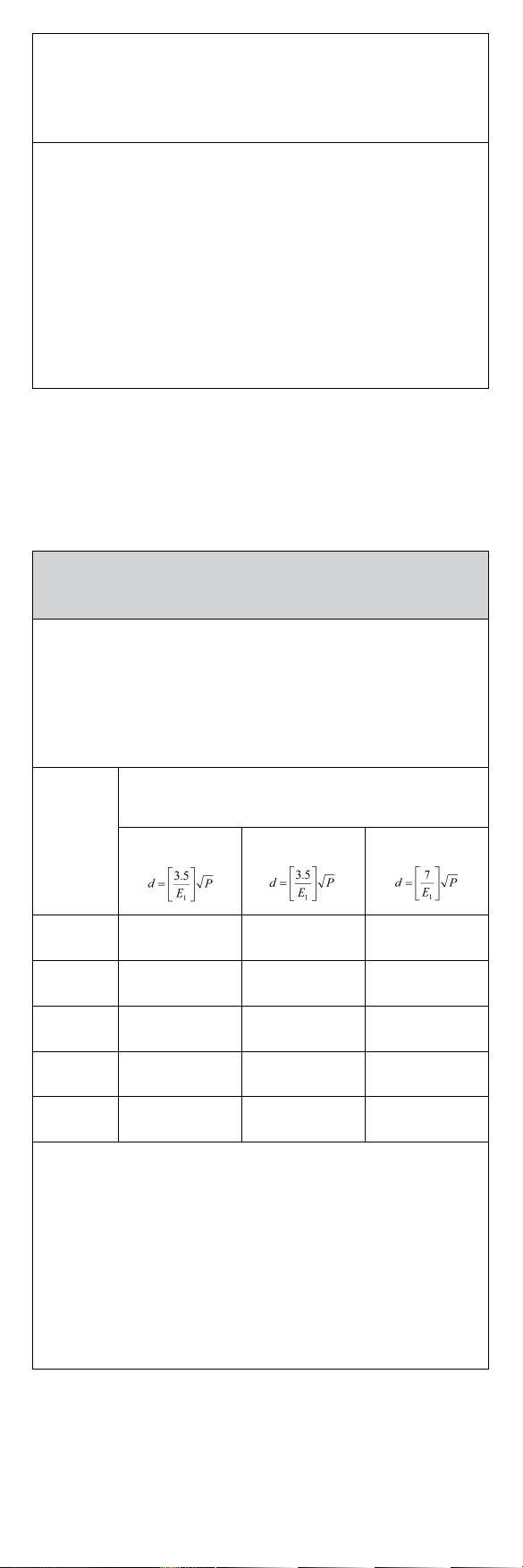
NOTES
• At 80 MHz and 800 MHz, the higher frequency range applies.
• These guidelines may not apply in all situations. Electromagnetic
propagation is affected by absorption and reection from structures,
objects and people.
a Field strengths from xed transmitters, such as base stations for radio
(cellular/cordless) telephones and land mobile radios, amateur radio, AM
and FM radio broadcast and TV broadcast cannot be predicted theoretically
with accuracy. To assess the electromagnetic environment due to xed
RF transmitters, an electromagnetic site survey should be considered. If
the measured eld strength in the location in which this Pulse Oximeter
is used exceeds the applicable RF compliance level above, this Pulse
Oximeter should be observed to verify normal operation. If abnormal
performance is observed, additional measures may be necessary, such as
reorienting or relocating this Pulse Oximeter.
b Over the frequency range 150 kHz to 80 MHz, eld strengths should be
less than 3 V/M.
Recommended separation distances between
portable and mobile RF communications equipment and the
Pulse Oximeter.
This Pulse Oximeter is intended for use in an electromagnetic environment
in which radiated RF disturbances are controlled.The user of this Pulse
Oximeter can help prevent electromagnetic interference by maintaining
a minimum distance between portable and mobile RF communications
equipment (transmitters) and this Pulse Oximeter as recommended
below, according to the maximum output power of the communications
equipment.
Rated
maximum
output
power of
transmitter
(W)
Separation distance according to frequency of
transmitter(m)
150 kHz to 80 MHz 80 MHz to 800 MHz 800 MHz to 2.7 GHz
0.01 0.058 0.035 0.07
0.1 0.18 0.11 0.22
10.58 0.35 0.7
10 1.83 1.10 2.21
100 5.8 3.5 7
For transmitters rated at a maximum output power not listed above, the
recommended separation distance d in metres (m) can be estimated using
the equation applicable to the frequency of the transmitter, where P is the
maximum output power rating of the transmitter in watts (W) according to
the transmitter manufacturer.
NOTES:
• At 80 MHz and 800 MHz, the separation distance for the higher
frequency range applies.
• These guidelines may not apply in all situations. Electromagnetic
propagation is affected by absorption and reection from structures,
objects and people.
GUIDANCE AND MANUFACTURER’S DECLARATION –
ELECTROMAGNETIC EMISSIONS –
FOR ALL EQUIPMENT AND SYSTEMS
GUIDANCE AND MANUFACTURER’S DECLARATION –
ELECTROMAGNETIC IMMUNITY –
FOR ALL EQUIPMENT AND SYSTEMS
RECOMMENDED SEPARATION DISTANCES BETWEEN
PORTABLE AND MOBILE
RF COMMUNICATIONS EQUIPMENT AND THE EQUIP-
MENT OR SYSTEM – FOR EQUIPMENT OR SYSTEM THAT
ARE NOT LIFE-SUPPORTING
Symbol Description
Refer to instruction manual/booklet
%SpO2Pulse oxygen saturation (%)
PRbpm Pulse rate (beats/minute)
The battery voltage is decient
(change the battery to avoid inaccurate
measurements)
1) No nger inserted
2) Inadequate signal
Battery positive electrode
Battery cathode
Exit standby mode
Adjust screen brightness
SN Serial number
Alarm inhibit
WEEE (2002/96/EC)
IP22 Ingress of liquids rank
Storage and Transport Temperature limitation
Storage and Transport Humidity limitation
Storage and Transport Atmospheric pressure
limitation
This side up
Fragile, handle with care
Keep dry
Recyclable
EMC DECLARATION
• The device is subject to special EMC precautions and it must be installed
and used in accordance with these guidelines.
• The electromagnetic eld can affect the device performance, so other
equipment used near the device must meet the corresponding EMC
requirements. Mobile phones, X-rays or MRI devices are possible
interference source, as they can emit high-intensity electromagnetic
radiation.
• Refer to above chapters for the minimum value of user’s physiological
signal. Inaccurate result will appear when the device operates with the
values lower than the descriptions in above chapter.
• The use of ACCESSORIES, transducers and cables other than those
specied, with the exception of transducers and cables sold by the
MANUFACTURER of the ME EQUIPMENT or ME SYSTEM as replacement
parts for internal components, may result in increased EMISSIONS or
decreased IMMUNITY of the ME EQUIPMENT or ME SYSTEM.
• This device should not be used adjacent to or stacked with other
equipment and that if adjacent or stacked use is necessary, it should be
observed to verify normal operation in the conguration in which it will
be used.
• Devices or systems may still be interfered by other equipment, even if
other equipment meets the requirements of the corresponding national
standard.
• Basic performance: SpO2 measured range: 70% - 100%, absolute error:
±2%; Pulse Rate measured range: 30 - 250 beats/minute, accuracy: ±2
beats/minute or ±2%, whichever is greater.
Guidance and manufacturer’s declaration – electromagnetic emission
This Pulse Oximeter is intended for use in the electromagnetic environment speci-
ed below. The user of this Pulse Oximeter should assure that it is used in such and
environment.
Emission test Compliance Electromagnetic
environment – guidance
RF emissions
CISPR 11 Group 1
This Pulse Oximeter uses RF energy only for its
internal function. Therefore, its RF emissions are very
low and are not likely to cause any interference in
nearby electronic equipment.
RF emissions
CISPR 11 Class B
This Pulse Oximeter is suitable for use in all
establishments, including domestic establishments
and those directly connected to the public
low-voltage power supply network that supplies
buildings used for domestic purposes.
Guidance and manufacturer’s declaration – electromagnetic immunity
This Pulse Oximeter is intended for use in the electromagnetic environment speci-
ed below. The user of this Pulse Oximeter should assure that it is used in such an
environment.
Immunity
test
IEC 60601
test level
Compliance
level
Electromagnetic environment -
guidance
Electrostatic
discharge (ESD)
IEC 61000-4-2
8 kV contact
15 kV air
8 kV contact
15 kV air
Floors should be wood, concrete or
ceramic tile. If the oor is covered
with synthetic material, the relative
humidity should be at least 30%. the
manufacturer may recommend the
ESD precautionary procedures to user.
Power frequency
(50Hz) magnetic
eld
IEC 61000-4-8
30A/m 30A/m
Power frequency magnetic elds
should be at levels characteristic
of a typical location in a typical
commercial or hospital environment.
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
3V (0.15MHz–
80MHz), 6V
(in ISM bands
between
0.15MHz and
80MHz)
10 V/m
80 MHz to
2.7GH
3V
(0.15MHz–
80MHz),
6V (in ISM
bands
between
0.15MHz
and 80MHz)
10 V/m
Portable and mobile RF
communications equipment
should be used no closer to
any part of the Pulse Oximeter,
including cables, than the
recommended separation
distance calculated from the
equation applicable to the
frequency of the transmitter.
Recommended separation
distance
80 MHz to 800 MHz
800 MHz to 2.7 GHz
Where P is the maximum output
power rating of the transmitter
in watts (W) according to the
transmitter manufacturer and d
is the recommended separation
distance in metres (m).
Field strengths from xed RF
transmitters, as determined
by an electromagnetic site
survey,ashould be less than
the compliance level in each
frequency range.b
Interference may occur in the
vicinity of equipment marked
with the following symbol:
KEY OF SYMBOLS
Note: This device and INSTRUCTION MANUAL may not contain all the following
symbols.
READING THE RESULTS
SpO2
Display range 0% - 99%
Measured range 0% - 100%
Accuracy 70% - 100%: ±2%;
0% - 69%: unspecied.
Resolution 1%
Pulse Rate (PR)
Display range 30 - 250 beats/minute
Measured range 30 - 250 beats/minute
Accuracy ±2 beats/minute or ±2%, whichever is greater
Resolution 1 beat/minute
Accuracy under low
perfusion
Low perfusion 0.4%:
SpO2: ±4%;
Pulse Rate: ±2 beats/minute
or ±2%, whichever is greater
Light interference Under normal and ambient light conditions,
the SpO2 deviation ≤ 1%
CLEANING & DISINFECTING THE PULSE OXIMETER
1. Remove batteries before cleaning.
2. Wipe nger chamber and exterior surface with a disinfecting solution of at
least 75% isopropyl alcohol.
• Do not immerse in water or liquid.
• Do not spray any liquid directly onto the device.
3. Allow to air-dry or wipe dry with a clean, soft cloth.
HIGH-PRESSURE STERILIZATION CANNOT BE USED ON THE DEVICE
MAINTAINING & STORING THE PULSE OXIMETER
1. Inspect this device regularly. Discontinue use if you notice visible damage.
2. Replace the batteries when low-battery indicator appears or device does
not turn on.
3.Remove the batteries before storage or when not in use for an extended
timeframe.
4. Make sure the device is clean and dry before storage.
5. Store this device in a dry, well-ventilated location away from extreme
temperatures.
DISPOSAL OF DEVICE & BATTERIES
At the end of its useful product life, dispose of/recycle this device and used
batteries following local regulations to avoid environmental pollution.
• DO NOT discard in household trash.
• NEVER dispose of batteries in re.
• Dispose of/recycle used batteries following local regulations to avoid
environmental pollution.
LIMITED 2-YEAR WARRANTY
This product* has a limited warranty of 2 years from the original date of
purchase against workmanship and defects in material. If under normal use,
your product fails to operate, please contact our customer service department
with proof of purchase. Star Elite Inc. may deny claims of damage caused by
misuse or modications of this product.
*Excluding accessories (Batteries, Lanyard & Carrying case)
FAQS / TROUBLESHOOTING
Q: The pulse oximeter does not turn on.
A: Check the batteries for correct installation. Replace both AAA batteries
when the battery indicator is low.
Q: The display screen turns off suddenly.
A: The device enters energy-mode when not in use for several seconds. Press
ON button.The batteries are low. Install 2 new AAA batteries.
Q: The display is not showing SP02and Pulse Rate.
A: Make sure the nger is fully inserted into the nger chamber. Remain still
while measuring. Nail polish or articial nails may interfere with an
accurate reading.

• Explosive hazard - DO NOT use this device in environment with
inammable gas such as anesthetic.
• DO NOT use this device during an MRI or CT scan, as the induced current
may cause burns.
• DO NOT take the information displayed on this device as the sole basis
for clinical diagnosis. This device is not intended as a replacement for
advice from a physician or medical professional.
• This device is NOT recommended for continuous monitoring.
• DO NOT use on a ngertip that has scar tissue or or that has any other
skin disorder as this may affect the measurement accuracy.
• DO NOT stare directly into the nger chamber of this device while it is
turned on as the infrared light may be damaging to the eyes.
• This device contains silicone, PVC, TPU, TPE and ABS materials, whose
biocompatibility has been tested in accordance with the requirements
in ISO 10993-1, and it has passed the recommended biocompatibility
test. Anyone with allergies to silicone, PVC, TPU, TPE or ABS should not
use this device.
• DO NOT use the included lanyard if you are allergic to nylon/polyester
material.
• DO NOT wrap the lanyard around the neck to avoid an accident.
• This device should be kept out of the reach of children/pets. When
not in use, store this device in a dry room and protect against extreme
moisture, heat, lint, dust and direct sunlight. Never place any heavy
objects on the pulse oximeter.
• DO NOT throw batteries into re.
• DO NOT discard in household trash.
• Only use recommended batteries.
• DO NOT use rechargeable batteries.
• DO NOT drop, disassemble or modify this device.
• DO NOT use this device if you think it is damaged or notice anything
unusual.
• This device comprises sensitive components and must be treated with
caution. Observe the cleaning, maintenance and operating conditions
described in this INSTRUCTION MANUAL.
• DO NOT perform service or maintenance while this device is in use.
• DO NOT touch the battery and the person at the same time.
• DO NOT use this device if it is damaged, degraded or loosened in any way.
• Continuous use of a damaged device may cause injury, improper
results, or serious danger.
• This device needs to be used and serviced in accordance with the
information provided in this INSTRUCTION MANUAL.
OVERVIEW
The oxygen saturation is the percentage of HbO2in the total Hb in the blood,
so-called the O2concentration in the blood, it is an important physiological
parameter for the respiratory and circulatory system.A number of diseases
related to the respiratory system may cause a decrease of SpO2in the blood.
APPLIED RANGE
This Pulse Oximeter is a non-invasive device intended for the spot-check
of arterial hemoglobin (SpO2) and the pulse rate of adults in home use
environments.This device is not intended for continuous monitoring. Solely for
use with sporting and aviation activities. Intended to monitor heart rate
during exercise.
ENVIRONMENTAL REQUIREMENTS
• Storage Environment
a) Temperature: -40°F - 140°F (-40°C - 60°C)
b) Relative humidity: ≤ 95%
c) Atmospheric pressure: 500 hPa - 1060 hPa
• Operation Environment
a) Temperature: 50°F - 104°F (10°C - 40°C)
b) Relative Humidity: ≤ 75%
c) Atmospheric pressure: 700 hPa - 1060 hPa
NOTE: BEFORE YOU BEGIN, WARM THE HANDS AND FINGERS BY
RUBBING THEM TOGETHER.
• Low blood perfusion can affect oximeter readings.
• 99% of reading errors are caused by cold extremities.
• Keep hand and nger still as movement may affect the reading.
• Allow at least 30 seconds for the measurement to stabilize.
• Nail polish and articial ngernails may cause false or no results.
Principle of this Pulse Oximeter is as follows: An experience formula of
data process is established taking use of Lambert Beer Law according to
Spectrum Absorption Characteristics of Reductive Hemoglobin (Hb) and
Oxyhemoglobin (Hb02) in glow & near-infrared zones. Operation principle of
the device is: Photoelectric Oxyhemoglobin Inspection Technology is adopted
in accordance with Capacity Pulse Scanning & Recording Technology, so that
two beams of different wavelength of lights can be focused onto human nail
tip through perspective clamp nger-type sensor. Then measured signal can
be obtained by a photosensitive element, information acquired through
which will be shown on screen through treatment in electronic circuits and
microprocessor.
1. Slide open the back cover and install 2 AAA batteries as shown.To avoid
damage, make sure the batteries align with the “+” and “–” symbols.
2. Replace the back cover.
Figure 4.
Attaching the connector loop
1. Thread the thin connector loop through the hole.
2. Insert the quick-release buckle through the loop.
3. Slide the lanyard clip into the quick-release buckle.
1. Gently squeeze to open the nger chamber.
2. Place nger fully into the chamber.
3. Press the ON button.
Fingertip Pulse Oximeter
Instruction Manual
Figure 1.
Operating principle
AAA
AAA
Figure 2. Fingertip Pulse Oximeter
AAA
AAA
AAA
AAA
Figure 5.
Put nger in position
WARNINGS
PRINCIPLE
TECHNICAL DATA
READ AND SAVE THESE INSTRUCTIONS. PLEASE READ THIS
INSTRUCTION MANUAL BEFORE USING THIS DEVICE. IF THE
USAGE IS NOT FULLY UNDERSTOOD, PLEASE DO NOT USE THIS
PULSE OXIMETER.
ATTENTION
• Use and store this device according the ENVIRONMENTAL
REQUIREMENTS section of this INSTRUCTION MANUAL.
• When the ambient temperature of the device changes too much, such
as when moving from one room at a lower temperature, to another
room at a higher temperature, allow the device to remain in the room
for 30 minutes before using.
• DO NOT use if this device is splashed by or immersed in water.
• High temperature, high pressure, gas sterilizing or immersion
disinfection for this device is not permitted. Refer to the CLEANING &
DISINFECTING section of this INSTRUCTION MANUAL.
• This device may not be suitable for all users. Measurement results
are for reference only. Contact your physician if you have or suspect a
medical condition.
• Data averaging and signal processing have a delay in the upgrade of
SpO2 data values. When the data update period is less than 30 seconds,
the time for obtaining dynamic average values will increase.
• DO NOT use external coloring agents (such as nail polish, articial nails,
or colored skin care products) on the test nger as this may affect the
measurement accuracy.
• Measurement accuracy may be impacted if the test nger:
-Is too cold. Warm the test nger up before using the device.
-Is too thin. Trying using a bigger nger such as the thumb or middle nger.
-Has a long ngernail. Trim the ngernail or switch to a nger with a
short nail.
• Refer to Figure 5 in this INSTRUCTION MANUAL for correct nger
position to use this device. Improper nger position may cause
inaccurate results.
• The light between the photoelectric receiving tube and the light-
emitting tube of this device must pass through the subject’s arteriole.
Make sure the optical path is free from any optical obstacles like
rubberized fabric, to avoid inaccurate results.
• Excessive ambient light may affect the measured results, such
as surgical light (especially xenon light sources), bilirubin lamp,
uorescent lamp, infrared heater and direct sunlight, etc. In order to
prevent interference from ambient light, make sure to place the sensor
properly and cover the sensor with opaque material.
• This device should not be placed on a limb with a blood pressure cuff,
arterial ductus or intraluminal tube.
• The measured value may be inaccurate during debrillation and in
a short period after debrillation, as it does not have a debrillation
function.
• This device has been calibrated before leaving factory.
• This device is calibrated to display functional oxygen saturation.
CLINICAL RESTRICTION
A. As the measurement is taken on the basis of arteriole pulse, substantial
pulsating blood ow of the user is required. For a user with weak pulse
due to shock, low ambient/body temperature, major bleeding, or use
of vascular contracting drug, the SpO2will decrease. In this case, the
measurement will be more sensitive to interference.
B. The measurement will be inuenced by intravascular staining agents (such
as indocyanine green or methylene blue), skin pigmentation.
C. The measured value may be seemingly normal for a user who has anemia
or dysfunctional hemoglobin (such as carboxyhaemoglobin (COHb),
methaemoglobin (MetHb) and sulfhaemoglobin (SuHb), however the user
may appear hypoxia. It is recommended to perform further assessment
according the clinical situation and symptoms.
D. Pulse oxygen only has a reference meaning for anemia and toxic hypoxia,
as some severe anemia patients still show better pulse oxygen measured
values.
E. Contraindication: no
FINGERTIP PULSE OXIMETER COMPONENTS
INSTALLING BATTERIES IN YOUR PULSE OXIMETER
ATTACHING THE LANYARD TO THE PULSE OXIMETER
MEASURING PULSE RATE AND BLOOD OXYGEN LEVELS
Optical sensor
Red light Wavelength: about 660 nm, optical output power: < 6.65 mW
Infrared light Wavelength: about 905 nm, optical output power: < 6.75 mW
Safety class Internally powered equipment, type BF applied part
International
Protection IP22
Working voltage DC 2.6 V - 3.6 V
Working current ≤ 25 mA
Power supply DC 3V (2 x AAA batteries)
Battery life Two batteries can work continually for approximately 24 hours
Dimension 2.2”(L) x 1.2”(W) x 1.3”(H)
57mm(L) x 31mm(W) x 32mm(H)
Weight About 1.8oz /51g (including batteries)
If you have any problems, please do not contact the store.
Contact our customer service at 1-877-383-6399
(8:30 am - 5:00 pm EST) Monday - Friday
or contact us at
Our customer service will be happy to assist you.
© 2021 Sunbeam Products, Inc.All rights reserved.
Distributed by Star Elite Inc., Montreal, Canada H3B 3X9.
SE004-071421
Fingertip Pulse Oximeter Model CMS50DL
Printed in China
Model : CMS50DL
Item: 16979 Figure 3.
Batteries Installation
Power Button
SpO² Display
Battery Level Indicator
Pulse Rate BPM (beats per minute)
Pulse Rate Bar Graph
Table of contents
Other Sunbeam Medical Equipment manuals

Sunbeam
Sunbeam GoHeat 804-RFL2P User manual

Sunbeam
Sunbeam 885 User manual

Sunbeam
Sunbeam 771 User manual

Sunbeam
Sunbeam Rest & Relieve FPO User manual

Sunbeam
Sunbeam 1501 User manual

Sunbeam
Sunbeam 720-905 User manual

Sunbeam
Sunbeam FlexTemp 901-825 User manual

Sunbeam
Sunbeam 835 Installation instructions