7
Guidelines and Manufacturer’s Declaration:
Electromagnetic Stability Interference
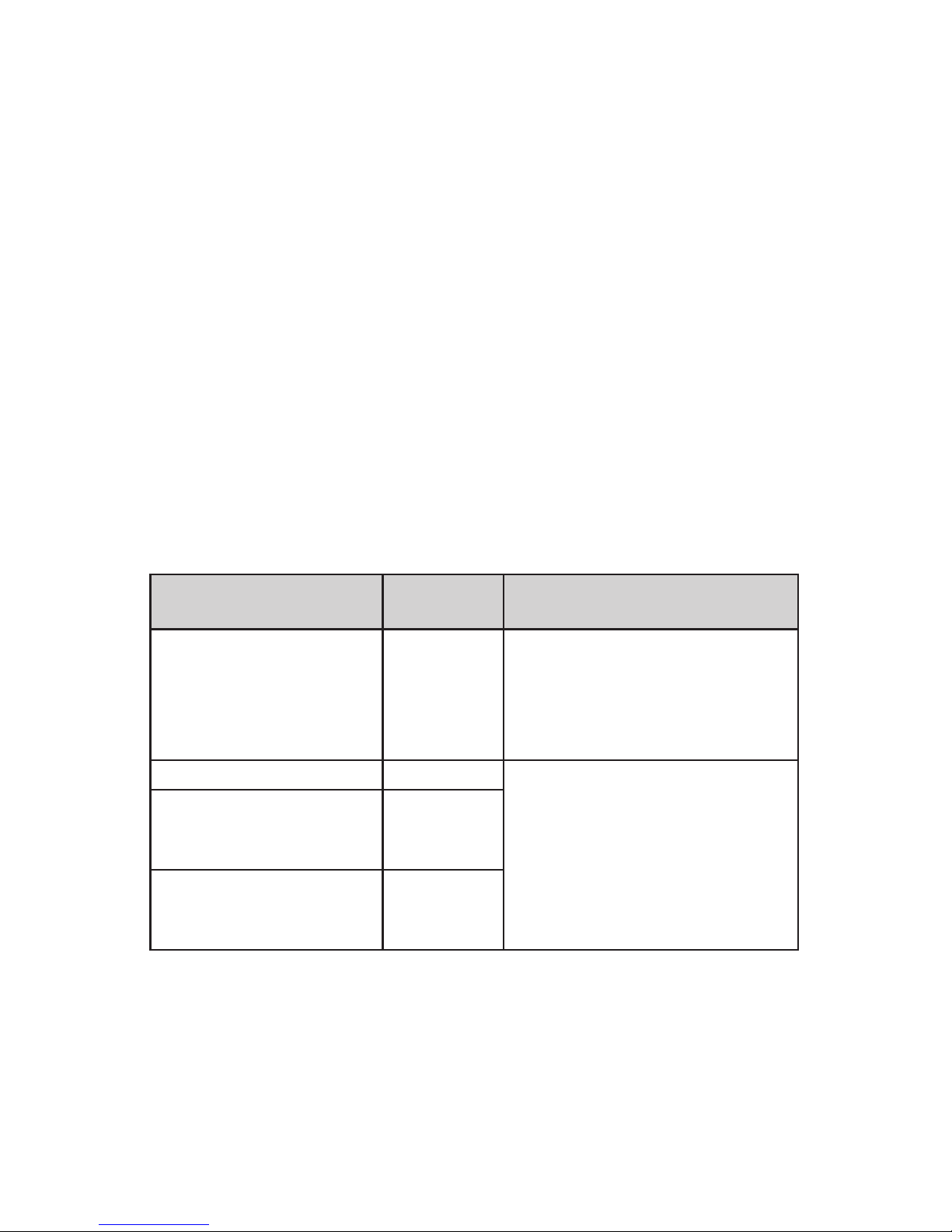
The iMRS one is intended for operation in an ELECTROMAGNETIC ENVIRONMENT
as indicated below. The customer or iMRS user should ensure that it is operated
in such an environment.
Interference
Immunity Tests
IEC 60601
Test Level
Com-
pliance
Level
Electromagnetic Environment - Guidelines
Conducted RF
interference
acc. to IEC
610004-4-6
Radiated RF
interference
acc. to IEC
610004-4-3
3 V effec-
tive value
150 kHz up
to 80 MHz
3 V/m
80 MHz up
to 2.5 GHz
3V Portable and mobile radio devices, includ-
ing their cables, should not be used at
a distance closer to the iMRS one than
recommended, which has been calculated
according to relevant equation for the trans-
mission frequency.
Recommended safe distance:
for 80 MHz up to 800 MHz
for 800 MHz up to 2.5 GHz
Where P is the rated power of the transmit-
ter in watts (W) according to the information
from the transmitter manufacturer and d is
the recommended safe distance in meters
(m). The eld strength of stationary radio
transmitters should be investigated locally
for all frequencies lower than the compli-
ance level 6
Interference is possible in proximity
to devices that bear the following
symbol.
NOTE 1 The higher frequency range applies at 80 MHz and 800 MHz.
NOTE 2 These guidelines may not apply in all cases. The expansion of electromagnetic
quantities will be affected by absorption and reection from buildings, objects and
people.
The eld strength of stationary transmitters, such as: the base stations of cordless
telephones and land mobile radio systems, amateur radio stations, AM/FM radio and
television transmitters; cannot be determined in advance with theoretic precision. A
study of the electromagnetic phenomena of the location should be considered in order
to determine the nature of the ELECTROMAGNETIC SURROUNDINGS in terms of sta-
tionary transmitters. If the eld strength measured at the location where the iMRS one
will be used exceeds the COMPLIANCE LEVEL mentioned above, the iMRS one should
be checked to verify its OPERATION in the manner intended. If unusual performance
characteristics are observed, additional measures may necessary, such as changing the
orientation or choosing a different location for the iMRS one.
The eld strength should be less than 3V/m over the frequency range 150 kHz to 80
MHz.
6) National footnote: User here is meant in the sense of RESPONSIBLE ORGANISATION.
d = 1,2√P
d = 1,2√P
d = 2,3√P