Instructions for Use
Indications
• Functional treatment of the knee during the pre- or
post-operative period, as well as the rehabilitation period
(including following moderate or severe cruciate ligament
and/or collateral ligament sprains).
• Conservative treatment of knee ligament injuries
and/or ruptures.
• Knee instability.
Contraindications
Patients with severe arterial insufficiency and/or spider veins
inducing skin at risk with the regular wearing of compression
orthosis or a known reaction or allergy to any of the material
ingredients
Warning and Precautions
Read all instructions prior to use. If increased pain, swelling,
change in sensation, or any adverse reactions are experienced
while using this product, immediately consult your medical
professional. This product is intended to be applied by
healthcare practitioner. For single patient use only.
Precautions
• Closely follow your healthcare professional’s prescription
and recommendations for use.
• Check the product is not damaged before use.
• Do not apply the product directly over broken skin or an
open wound without an appropriate dressing.
•
sensations, remove the product and seek the advice of a
healthcare professional.
• In the event of a change in product performance, contact
the healthcare professional who prescribed or supplied the
product.
• This product is intended for the treatment of a given
condition. Its duration of use is limited to this treatment only.
• For hygiene and performance reasons, do not re-use the
product for another patient.
• Do not apply the device in direct contact with a greasy
substance (ointment, cream, etc.).
• Remove the product before any imaging exams.
• Store at room temperature, preferably in its original packaging.
• The product should be disposed of as per local regulations.
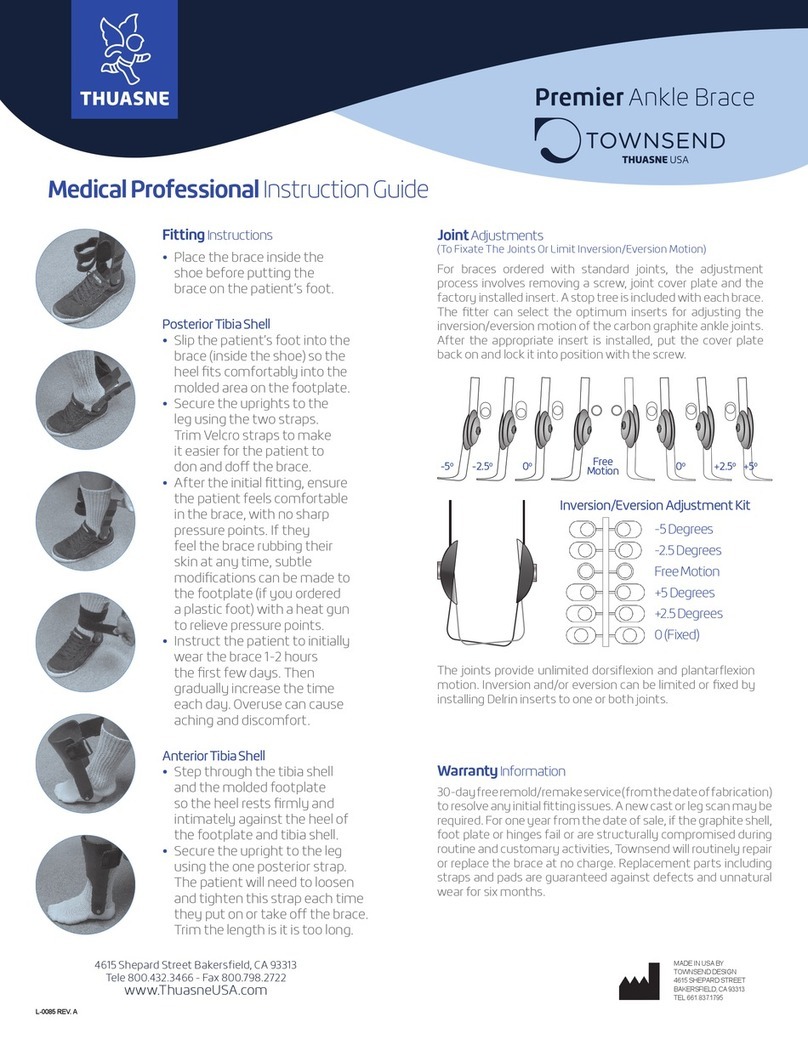
Description
• Purpose: the product is aimed at patients with a moderate
to severe knee ligament injury and/or in postoperative
treatment. The patient’s thigh and calf circumferences
must correspond to the fitting range.
• Properties/Mechanism of action: Stabilization of knee
joint ligaments provided by the rigid hinged side uprights.
- Extension can be adjusted to 10° and 20°.
- Flexion can be adjusted to 0°, 45°, 60° and 90°.
• The product is available in 4 forms: 2 versions
(open and closed), each available in 2 lengths (short and
long) to meet the needs of patients (example of open long
version in figure A and closed short version in gure B).
Each version is available in 7 sizes.
• The product is composed of:
- a fabric part consisting of a 3D knit at the front and a
thin, elastic knit at the back;
-
- the TM5+ hinge mimicking the knee’s natural
- 2 anterior half-straps and 2 posterior half-straps
- 4 anterior half-straps and 3 posterior half-straps
- a patellar insert to enable correct positioning of the
•
be used without tools.
• The design of the frame enables limitation of extension
to 0° by default.
•
the hinge
of the knee brace, is included in the packaging.
• The product is made of aluminum, stainless steel,
polyamide, elastane, polyester, synthetic rubber,
polyoxymethylene, TPU, and PVC.
Unwanted side eects
• This device can cause skin reactions (redness, itching,
blistering, burns, blisters, etc.)
• Any serious incidents occurring related to the device
should be reported to the manufacturer and to the
competent authority of the country in which the user and/or
patient is resident.
Brace Components & Sizing
Size
Circumference Measurement At:
6" Above
Knee Center Knee Center 6" Below
Knee Center
XS
SM
MD
LG
XL
2XL
3XL
Components
1. Patella buttress
2. Wrap opening
3. Upper straps
4. Lower straps
5. Posterior straps
6. TM5 hinge and metal uprights/stays
7.
9. Flexion/Extension Pins (six included)
GenuStart ROM
Hinged Ligament Knee Brace With Flexion/Extension Control
Also available in short
length and/or sleeve
style application
Figure A
Figure B
Remove stays and
hand wash 30°C
with mild detergent
Do not Iron
Do not
tumble
Do not
dry clean
Lay sleeve
flat to dry
Do not
bleach
Do not submerge
uprights and
hinge in water
single patient,
multiple use
L-0133 Rev. A