1
TABLE OF CONTENTS
Paragraph Page No.
1GENERAL................................................................................................................3
1.1 Manufacturer and US Official Correspondence Information......................3
1.2 Intended Use..................................................................................................3
1.3 Intended Users ..............................................................................................3
1.4 Incoming Inspection .....................................................................................3
1.5 Warranty........................................................................................................3
1.6 Warranty Statement ......................................................................................4
2TECHNICAL DATA................................................................................................5
2.1 Introduction ..................................................................................................5
2.2 Storage Conditions........................................................................................6
2.3 Operating Conditions....................................................................................6
2.4 Construction..................................................................................................6
2.5 Directives and Standards ..............................................................................7
2.6 Utilities ..........................................................................................................8
2.7 Waste Water Disposal ...................................................................................8
2.8 Environment Emission Information.............................................................8
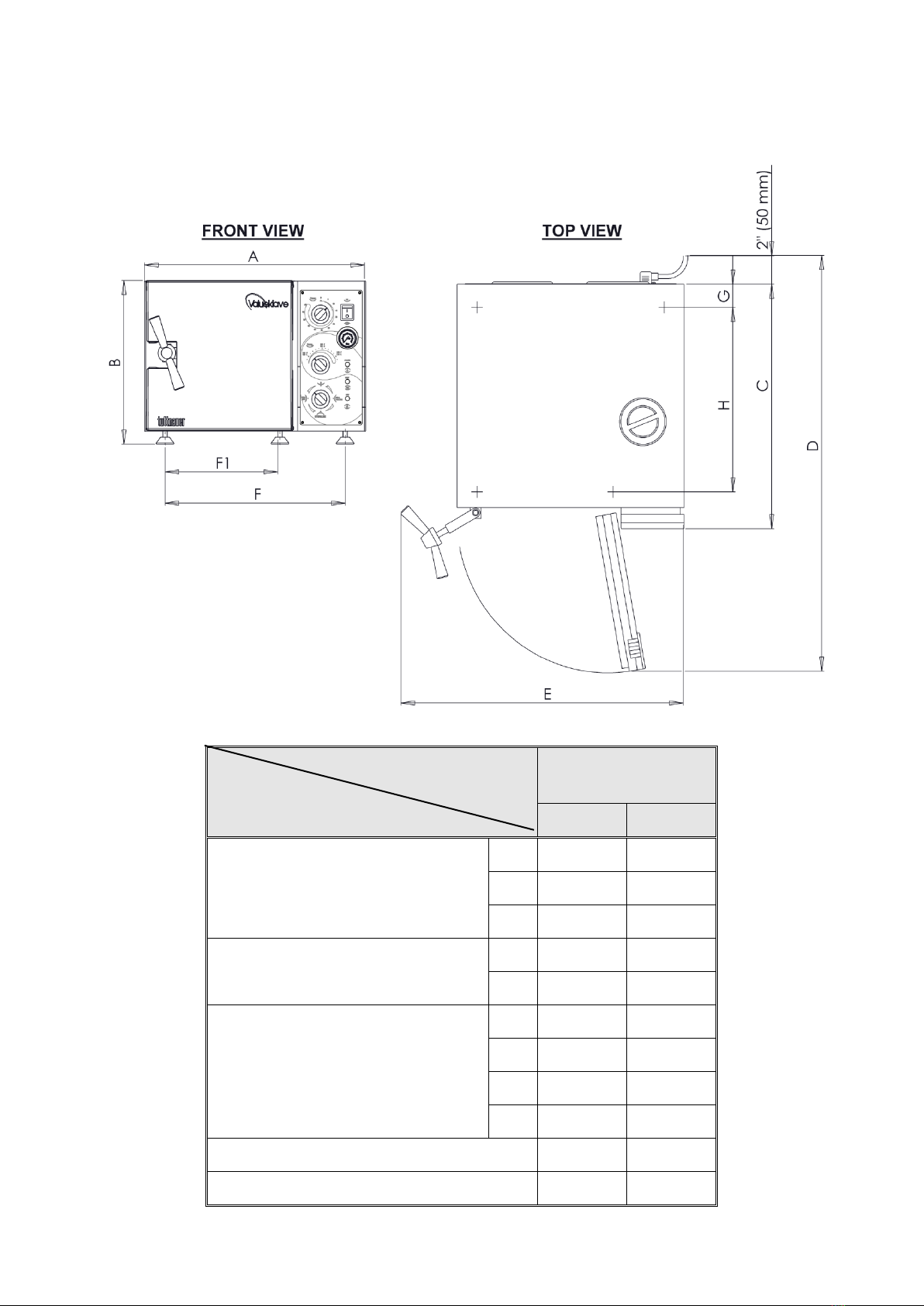
2.9 Dimensions, Models 1730 .............................................................................9
2.10 Dimensions, Models 2340, 2540 .................................................................10
2.11 Dimensions, Models 3140 ...........................................................................11
2.12 Dimensions, Models 3850, 3870 .................................................................12
2.13 Technical Specifications .............................................................................13
2.14 Electrical Data ............................................................................................14
2.15 Maximum Solid Load Sizes.........................................................................14
2.16 Symbols Description....................................................................................15
2.17 Water Quality ..............................................................................................17
3DESCRIPTION OF COMPONENTS....................................................................23
3.1 Control Panel ..............................................................................................23
3.2 Other Components ......................................................................................23
4INSTALLATION INSTRUCTIONS......................................................................24
4.1 Electrical .....................................................................................................24
4.2 Setup............................................................................................................24
4.3 Lifting and Carrying...................................................................................25
5PREPARATION BEFORE STERILIZATION .....................................................26
6OPERATION .........................................................................................................30
6.1 Loading and Unloading the Device ............................................................30
6.2 Fill the Water Reservoir..............................................................................30
6.3 Sterilization Time Table..............................................................................32
7SERVICE AND MAINTENANCE INSTRUCTIONS...........................................35
7.1 Preventive and Scheduled Maintenance.....................................................35
7.2 Draining the Reservoir................................................................................36
7.3 Cleaning the Air Jet....................................................................................37
7.4 Replacing the Door Gasket .........................................................................38
7.5 Checking the Safety Valve ..........................................................................39