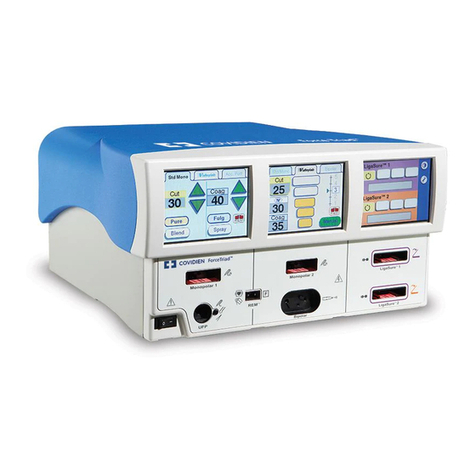
Safety
1-4 SurgiStat II-20 User’s Guide
Warnings
Warning
Hazardous Electrical Output This equipment is for use only by trained,
licensed physicians.
Danger: Fire/Explosion Hazard Do not use the SurgiStat II electrosurgical
generator in the presence of flammable anesthetics.
Fire/Explosion Hazard The following substances will contribute to increased
fire and explosion hazards in the operating room:
• Flammable substances (such as alcohol based skin prepping agents and
tinctures)
• Naturally occurring flammable gases that may accumulate in body cavities
such as the bowel
• Oxygen enriched atmospheres
• Oxidizing agents (such as nitrous oxide [N2O] atmospheres)
The sparking and heating associated with electrosurgery can provide an ignition
source. Observe fire precautions at all times. When using electrosurgery in the
same room with any of these substances or gases, prevent their accumulation or
pooling under surgical drapes, or within the area where electrosurgery is
performed.
Electric Shock Hazard Connect the power cord to a properly polarized and
grounded power source with the frequency and voltage characteristics that match
those listed on the back of the unit. Do not use power plug adapters.
Electric Shock Hazard Always turn off and unplug the generator before
cleaning.
Fire Hazard Do not use extension cords.
Patient Safety Use the generator only if the self-test has been completed as
described. Otherwise, inaccurate power outputs may result.
Failure of the high frequency electrosurgical equipment could result in an
unintended increase of output power.
The instrument receptacles on this generator are designed to accept only one
instrument at a time. Do not attempt to connect more than one instrument at a
time into a given receptacle. Doing so will cause simultaneous activation of the
instruments.
Use the lowest output setting necessary to achieve the desired surgical effect.
Use the active electrode only for the minimum time necessary in order to reduce
the possibility of unintended burn injury. Pediatric applications and/or procedures
performed on small anatomic structures may require reduced power settings. The
higher the current flow, and the longer the current is applied, the greater the
possibility of unintended thermal damage to tissue, especially during use on
small structures.