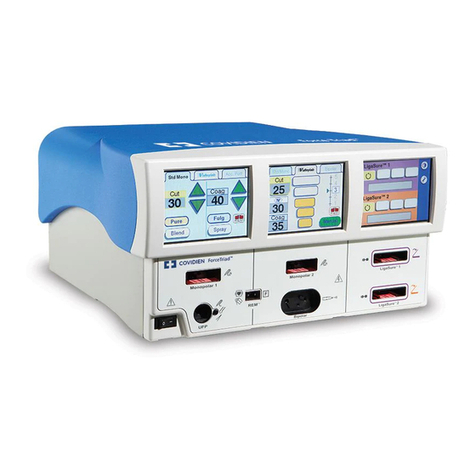
1-2 Force 2 User’s Guide
General
Fire/Explosion
Warning
Always use the lowest output setting necessary that achieves the desired surgical
effect. The active electrode should be utilized only for the minimum time
necessary in order to lessen the possibility of unintended burn injury. Pediatric
applications and/or procedures performed on small anatomic structures may
require reduced power settings. The higher the current flow and the longer the
current is applied, the greater the possibility of unintended thermal damage to
tissue, especially during use on small structures.
Use electrosurgery with caution in the presence of internal or external
pacemakers. Interference produced with the use of electrosurgical devices can
cause devices such as a pacemaker to enter an asynchronous mode or can block
the pacemaker entirely. Consult the pacemaker manufacturer or hospital
cardiology department for further information when use of electrosurgical
appliances is planned in patients with cardiac pacemakers.
Valleylab recommends against the use of laparoscopic surgery on pregnant
patients.
Do not use electrosurgical equipment unless properly trained to use it in the
specific procedure being undertaken. Use by physicians without such training
has resulted in serious, unintended patient injury, including bowel perforation and
unintended, irreversible tissue necrosis.
Hazardous Electrical Output: This equipment is for use only by trained, licensed
physicians.
Caution
This equipment is capable of producing a physiological effect.
Read the warnings, cautions, and instructions provided with this generator before
using.
For surgical procedures where the current could flow through delicate parts of the
body, the use of bipolar techniques may be desirable in order to avoid unwanted
coagulation.
Danger
Explosion Hazard: Do not use in the presence of flammable anesthetics.