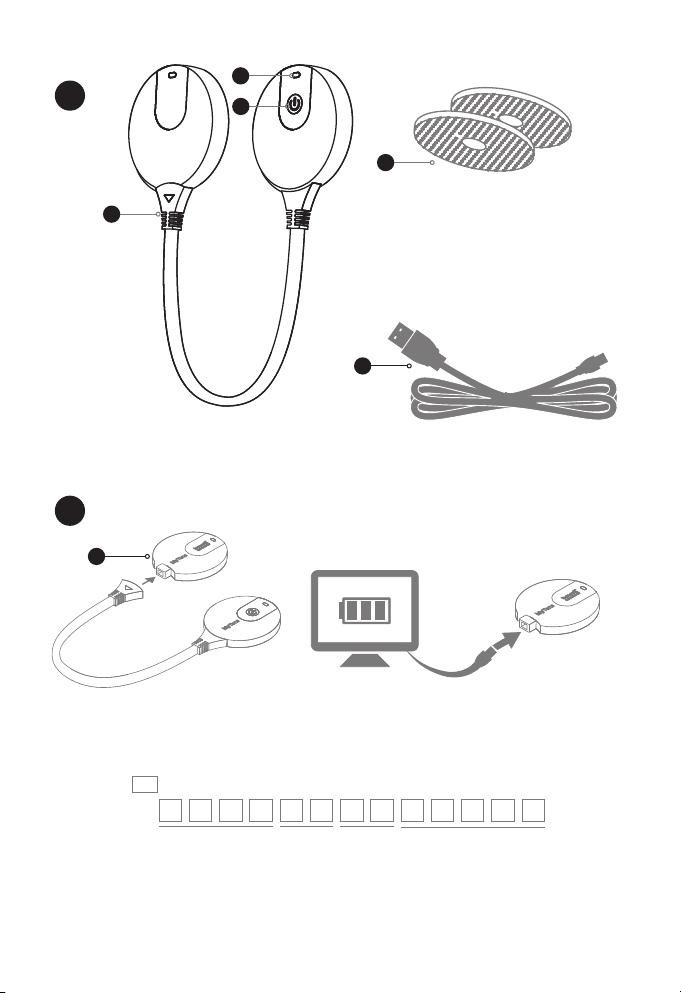
BewellConnect - BW-TS1 - User Manual - 122016V1 9
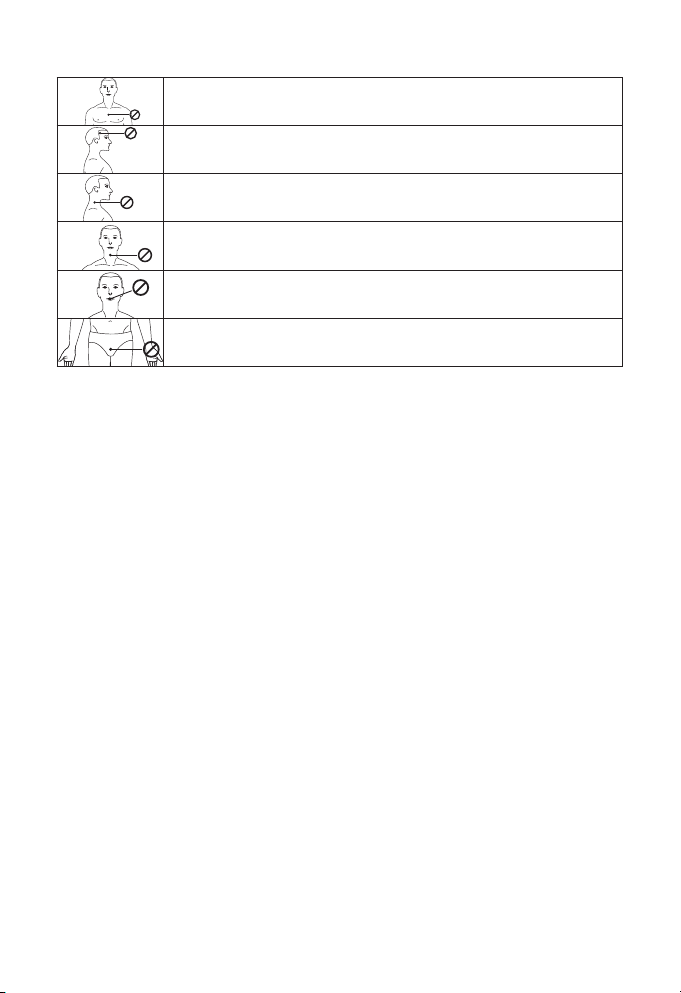
Do not treat the the following parts or areas :
Do not apply stimulation on your chest, the introduction of electric cur-
rent on this area can cause heart rhythm disturbances, with a risk of death.
Its eects on the brain are unknown. Stimulation to the head or on each
side of the skull must be avoided.
Stimulation on the sides of the neck or on the carotid artery can cause seri-
ous adverse eects on your blood pressure or your heart rhythm.
Stimulation on the front of the neck can cause severe muscle spasms that
can block your airways and cause breathing diculties.
Do not place the electrodes on the inside of body cavities, such as in the
mouth. This device is only designed for external application.
Do not place the electrodes on the genitals. They could stimulate inappro-
priate muscles or organs.
- Do not position the electrodes on broken or injured skin, or that which is dirty or unhealthy.
Skin with irritation, sores or other lesions can lead to the injection of too much current on the
area, which can cause burns.
- Do not place the electrodes near cancerous lesions because this may have a negative impact
on these injuries.
- Do not place the electrodes on skin areas on which sensations are not normal. You may burn
yourself due to a lack of perception of the high intensity of the current.
- Do not put the electrodes on areas that are swollen, red, infected or inamed or on skin rashes
(eg. phlebitis, thrombophlebitis and varicose veins). Stimulation should not be performed on
areas of thrombosis or thrombophlebitis because it can promote the circulation and lead to a
greater risk of embolism.
- Do not put the electrodes on redness or open wounds. Open wounds may lead to applying too
much current on the area, causing burns. They can also further the penetration of substances
from the electrode into the skin.
-
Do not make sudden movements during a session. This could cause a dysfunction of the device.
Do not use the device in the following conditions :
- Do not use the device if you are connected to high frequency surgical equipment. This could
lead to burns on the skin under the electrodes and damage the device.
- Do not use the electro-stimulator if you are monitored by a doctor and you have not consulted
him before using it.
- In the case of internal bleeding due to impacts or injury, do not use the device.
- Do not use electrical muscle stimulation if muscle contraction can disrupt the healing process.
If the tendon or the muscle is torn, a muscle contraction can aggravate the tear. This can also
occur after recent surgery or after an acute trauma or fracture. A muscle contraction can also
aggravate the symptoms of tendonitis.
- Do not use the device while driving, operating machinery or any other activity during which
the electrical stimulation may lead to a risk of injury.
- Do not use the device if you are subject to falling asleep during the session, as this may cause
you to feel pain too late. If using at the time of going to bed, set the timer so that the device
does not turn itself o automatically.
- Never use MyTens in contact with water (in the bathroom, in the shower or in the pool, etc. )
because this increases the risk of an electric shock and skin burns.
2. TRANSCUTANEOUS NEUROSTIMULATION INFORMATION
My Tens is a device designed for adults to alleviate acute post surgical pain or low to moder-
ate chronic pain. It works on the principle of transcutaneous electrical neurostimulation (TENS),
which enables you to relieve pain and soothe muscle tension. This device also helps to promote
venous return and to strengthen the muscle mass. The device has been specially designed to be
used at home.Transcutaneous electrical neurostimulation (TENS) is a non-analgesic drug therapy
without secondary eects, used by doctors and physical therapists for more than 30 years. The
electrodes are placed on dierent areas of the body and transmit a low electrical current to the
nerves. This stimulation brings the body to produce and disseminate endogenous analgesic-
painkilling substances (endorphins, enkephaline) whose function is to anesthetize the pain.
In case of pain or muscle tension, mini-electrical impulses in the nervous tissue may block the
transmission of pain signals to the nervous system and trigger the release of endorphins. You
can choose your program among 11 preset TENS protocols on the MyTens application.
For the strengthening of your muscle mass, you can select a program among 8 protocols of elec-
trical muscle stimulation (EMS). MyTens sends, via the electrodes placed on the area to stimu-
late, electrical pulses causing a muscle contraction which strengthens the muscle.
It is controlled from the MyTens application. A diagram of the human body with treatment
zones helps you to position the electrodes correctly (see paragraph 10).
The MyTens application is available on the BewellConnect® website or as a free app in the Apple
store or Google Play store.
Contraindications
- On a damaged skin area , desensitized to the electrodes or the intermediate paste.
- On an anterior area of the neck (near the carotid) or sides.
- During an electrocardiogram or an electroencephalogram.
- In case of heart disease
- If you have epilepsy
- If you wear a pacemaker, a debrillator or any other active implantable or metallic medical
device (for example a drug administration system). In these conditions, use could cause an
electric shock, interference, burns or even death.
- If you are pregnant, during the rst trimester. The eects of TENS on the development of the
fetus are not yet known. During pregnancy, do not use the device on the uterus or abdomen
to avoid triggering of contractions. Always consult your doctor or midwife if you are pregnant
and intend to use the device.
- If you suer from venous thrombosis or high blood pressure, or thrombophlebitis. Using the
electrical muscle stimulation (EMS) device could, in this case, cause a blood clot.
- In case of cognitive impairment