BewellConnect - BW-TSX/BW-TS1 - User Manual - 11-2017v2 BewellConnect - BW-TSX/BW-TS1 - User Manual - 11-2017v28 9
- Possible effects from RF sources in the vicinity of the device (e.g, electromagnetic security
systems, cellular telephones, RFID or other in- band transmitters).
- Do not operate this unit in an environment where other devices are used that intentionally
radiates electromagnetic energy in an unshielded manner, such as MRI, RFID, metal detectors,
and electronic article surveillance (EAS) anti-theft systems.
- Do not use the device if it is closer than 12 in (30.5 cm) to wireless communication equipment,
such as wireless home network routers, cell phones, cordless phones and their base stations,
and walkietalkies. The electromagnetic interference of this wireless communication
equipment may prevent the device from operating properly.
- This device is not designed to be used by persons (including children) whose physical, sensory
or mental capabilities are reduced, or persons without experience or knowledge, unless they
have been able to benefit, by the intermediary of a person responsible for their safety, from
supervision or prior instructions concerning use of the device. It is possible that they will not
be able to use it in accordance with the instructions of this user manual and be disturbed by
the treatment.
- Do not modify the device or the electrodes. This could cause a malfunction.
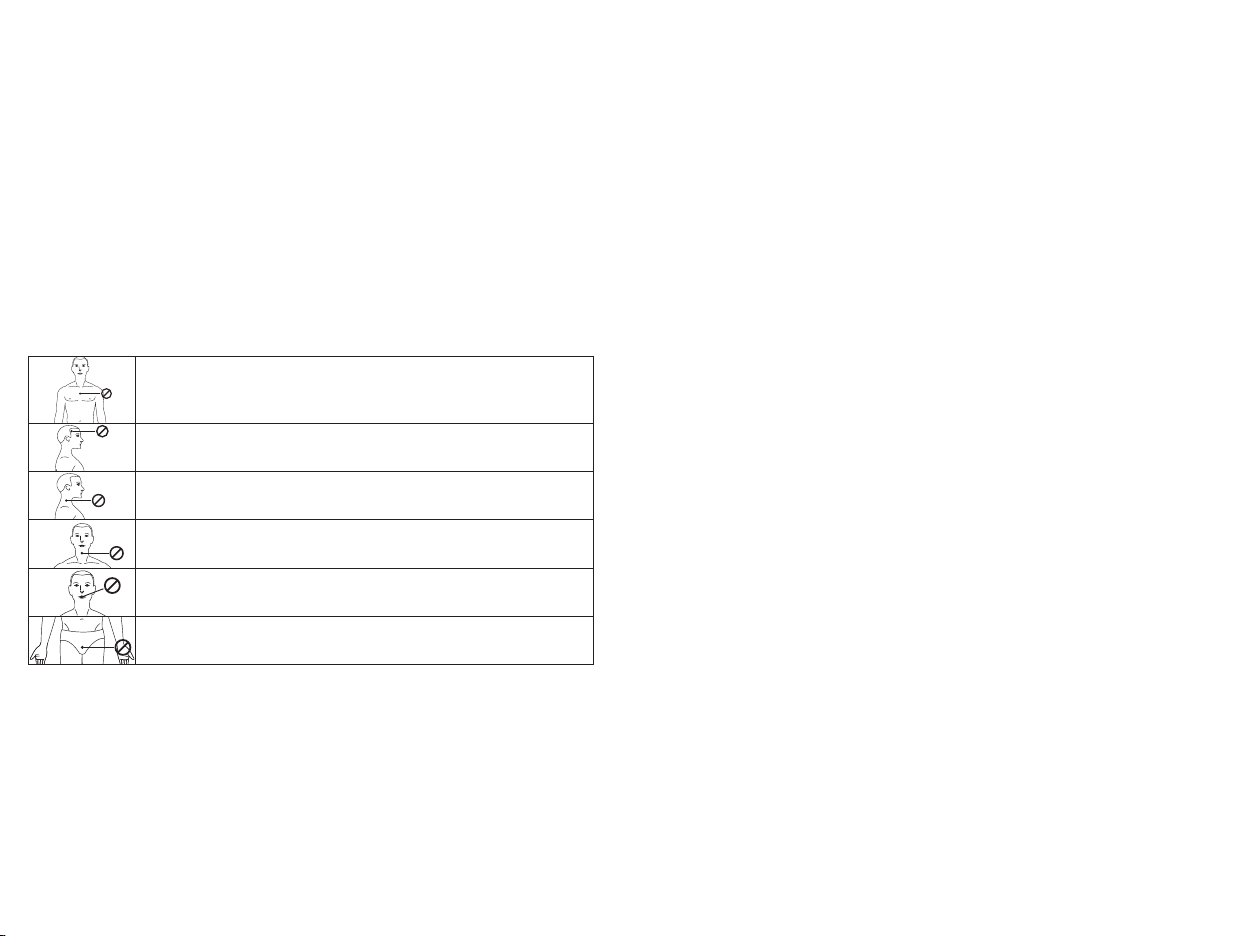
Do not treat the the following parts or areas:
Do not apply stimulation on your chest, the introduction of electric
current on this area can cause heart rhythm disturbances, with a risk of
death.
Its effects on the brain are unknown. Stimulation to the head or on each
side of the skull must be avoided.
Stimulation on the sides of the neck or on the carotid artery can cause
serious adverse effects on your blood pressure or your heart rhythm.
Stimulation on the front of the neck can cause severe muscle spasms that
can block your airways and cause breathing difficulties.
Do not place the electrodes on the inside of body cavities, such as in the
mouth. This device is only designed for external application.
Do not place the electrodes on the genitals. They could stimulate
inappropriate muscles or organs.
- Do not position the electrodes on broken or injured skin, or that which is dirty or unhealthy.
Skin with irritation, sores or other lesions can lead to the injection of too much current on the
area, which can cause burns.
- Do not place the electrodes near cancerous lesions because this may have a negative impact
on these injuries.
- Do not place the electrodes on skin areas on which sensations are not normal. You may burn
yourself due to a lack of perception of the high intensity of the current.
- Do not put the electrodes on areas that are swollen, red, infected or inflamed or on skin rashes
(eg. phlebitis, thrombophlebitis and varicose veins). Stimulation should not be performed on
areas of thrombosis or thrombophlebitis because it can promote the circulation and lead to a
greater risk of embolism.
- Do not put the electrodes on redness or open wounds. Open wounds may lead to applying too
much current on the area, causing burns. They can also further the penetration of substances
from the electrode into the skin.
- Do not make sudden movements during a session. This could cause a dysfunction of the
device.
- If batteries leak and come into contactr with the skin or eyes, wash immediately with copious
amounts of water.
- Do not apply stimulation over, or in proximity to, cancerous lesions.
- Apply stimulation only to normal, intact, clean, healthy skin.
Do not use the device in the following conditions:
- Do not use the device to children or infants, because the device has not been evaluated for
pediatric use.
- Do not use the device to pregnant women, because the safety of electrical stimulation during
pregnancy has not been established.
- Do not use the device to persons incapable of expressing their thoughts or intentions.
- Do not use the electro-stimulator if you are monitored by a doctor and you have not consulted
him before using it.
- In the case of internal bleeding due to impacts or injury, do not use the device.
- Do not use the device while driving, operating machinery or any other activity during which
the electrical stimulation may lead to a risk of injury.
- Do not use the device if you are subject to falling asleep during the session, as this may cause
you to feel pain too late. If using at the time of going to bed, set the timer so that the device
does not turn itself off automatically.
- Never use MyTens in contact with water (in the bathroom, in the shower or in the pool, etc. )
because this increases the risk of an electric shock and skin burns.
PAIN MANAGEMENT WARNINGS:
- If your pain does not improve, becomes seriously chronic or severe, or continues for more than
five days, stop using the device and consult with your physician.
- The mere existence of pain functions as a very important warning telling us that something
is wrong. Therefore, if you suffer from any serious illness, consult your physician in order to
confirm that it is advisable for you to use this device.
WARNINGS AND PRECAUTIONS REGARDING THE ELECTRODE PADS:
- If you experience any skin irritation or redness after a session, do not continue stimulation in
that area of the skin.
- Electrode pads should not touch each other when placed onto your skin.
2.3 CAUTION
- Do not bend or fold because the electrode pad may not function properly. Place the electrode
pads onto the plastic film and then store into the sealed package when not in use.
- Do not apply ointment or any solvent to the electrode pads or to your skin because it will
disrupt the electrode pads from functioning properly.