Introduction
Intended Use
Pulsate™ is indicated for the prevention and treatment of pressure injuries, the treatment of severe or
extensive burns, and to aid in circulation.
Purpose of the Device
Pulsate™ is a therapeutic support surface that provides immersion for pressure redistribution. It offers
two therapy modes: pulsation and static (immersion), both with low air loss.
Pulsate can be used for patients with spinal cord injury once the acute injury has been stabilized and
these patients have been accessed and cleared by the appropriate physician. Pulsate is not
recommended for use by patients with unstable spinal fractures.
The selection of a pressure redistribution surface should be based on each individual patient’s clinical
condition, diagnosis and/or co-morbidities. The choice and use of a support surface is one factor in a
holistic program of wound prevention and treatment.
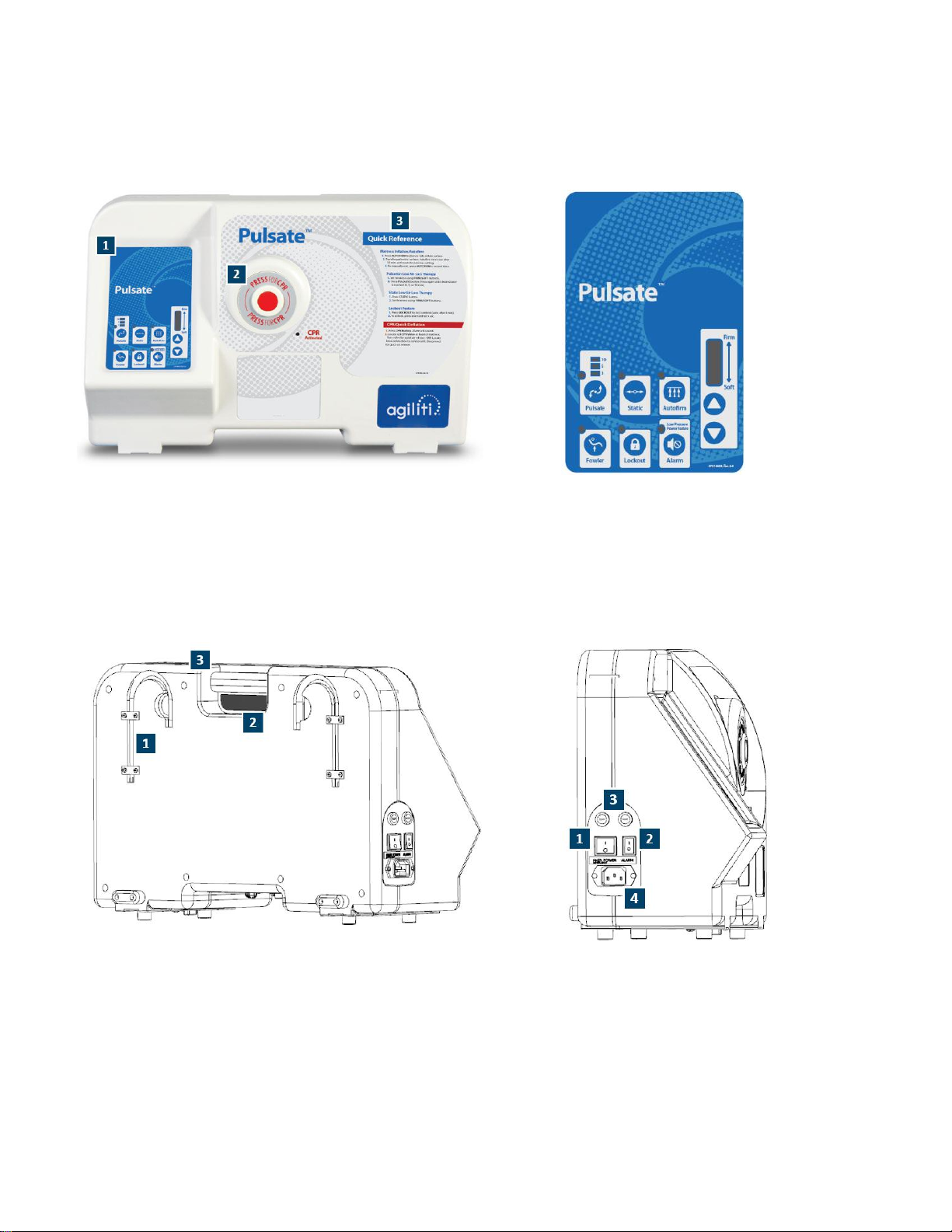
Device Information
Pulsate™ Low Air Loss Mattress System is comprised of a specialized inflatable air bladder (air
mattress) and an electrically powered control unit.
Pulsate is a specialized, multi-cell air mattress sized to fit a standard hospital bed frame. The air
mattress serves as a replacement to the original mattress and is equipped with 6 air tubes with
connectors that connect to the control unit. The control unit is a self-contained, totally enclosed
module that hangs by hinged hooks on the footboard of the bed.
If the footboard is too wide, hang a control unit hanger bar on the footboard. If no hanger bar is
available, use best judgment on control unit placement. Do not place control unit under the bed.
The control unit is provided with a detachable hospital grade electrical cord and has a control panel
with selector switches and indicator lights. The switches and indicators are protected under a flexible
membrane to keep liquids out spills and make cleaning easier. Inside the control unit is a variable
output blower and manifold that allow the air mattress to operate in a static (immersion) mode or
provide pulsation pressure variations within the mattress. There is also a printed circuit board which
operates the electrical controls.
General Information
—
Authorized user is defined as an adult.
—
Read and completely understand the manual before use.
—
DO NOT modify this equipment without authorization from the manufacturer.
—
Only use Agiliti accessories that are specific to Pulsate.
—
At certain intervals, maintenance of this product is required and must be performed by authorized personnel
only.
—
Retain this manual for future use and maintenance.
—
Patients, or users, should be risk assessed to ensure they are able to understand this manual and operate the
Pulsate safely without risk to themselves or others.
Symbols, definitions, and fonts are used throughout this manual to aid user readability and
understanding of content.
—
Standard Text: Used for regular information.
—
Bold Text: Emphasizes a word or phrase.
—
NOTE
: Sets apart special information or important instruction clarification.
IFU0000072 Pulsate User Manual