Allergan Natrelle User manual

®
®
®®
®®
®®®
®
®®®
®
®®®
®
®
®®®
® ®
®
®®
®®®
®
®®®
®
®®®®
®®®®
®
®®®
®
®®®
®
®®®®
®®®®
®
®®®
®
®®®
®
®®®
®
®®®®
®
®®®
®
®®®
®
®®®
®
®®®
®
®®®®
®
®
®®®
®
®®®
®
® ®
®
®
®
®®
®®
™
®
®
®
®
BREAST IMPLANTS AND TISSUE EXPANDERS
IMPLANTS MAMMAIRES ET EXPANSEURS TISSULAIRES
BRUSTIMPLANTATE UND GEWEBEEXPANDER
PROTESI MAMMARI ED ESPANSORI TISSUTALI
IMPLANTES MAMARIOS Y EXPANSORES TISULARES
IMPLANTES MAMÁRIOS E EXPANSORES TECIDULARES
BORSTIMPLANTATEN EN WEEFSELEXPANDERS
BRÖSTIMPLANTAT OCH VÄVNADSEXPANDERS
BRYSTIMPLANTATER OG VEVSEKSPANDERE
BRYSTIMPLANTATER OG VÆVSEKSPANDERE
RINTAIMPLANTIT JA -KUDOSVENYTTIMET
ΕΜΦΥΤΕΥΜΑΤΑ ΜΑΣΤΟΥ ΚΑΙ ΔΙΑΣΤΟΛΕΙΣ ΙΣΤΩΝ
MEME İMPLANTLARI VE DOKU GENİŞLETİCİLERİ
ГРУДНЫЕ ИМПЛАНТАТЫ И ТКАНЕВЫЕ ЭКСПАНДЕРЫ
ІМПЛАНТАТИ ТА ТКАНИННІ ЕКСПАНДЕРИ МОЛОЧНОЇ ЗАЛОЗИ
EN
FR
DE
IT
ES
PT
NL
SV
NO
DA
FI
EL
TR
RU
UK
*3441-01*
ALLERGAN
Marlow International, Parkway, Marlow,
Bucks, SL7 1YL, United Kingdom.
T. +44 (0)1628 494456
F. +44 (0)1628 494956
E. Productsuppor[email protected]
www.allergan.com
NATRELLE® and ALLERGAN® and the Representation
of an Eye Logo are registered trademarks of
Allergan, Inc. All other trademarks mentioned herein
are property of their respective owners.
© 2015 Allergan. All rights reserved.
3441-01 (L3441 Rev.03) 08/2015
11/1997
Release Date: 29 Sep 2015 00:07:57 GMT -07:00
Expires one day from 21 Dec 2015
Effective
1
DESCRIPTION
Natrelle®breast implants and tissue expanders are designed for use in
augmentation and reconstruction mammoplasty. All Natrelle®implants and
expanders are constructed of a silicone elastomer shell and are latex free.
- Natrelle®single lumen gel-filled breast implants are pre-filled with cohesive
silicone gel designed to simulate natural breast tissue.
- Natrelle®saline-filled breast implants are filled with saline at the time of surgery.
- Natrelle®133 tissue expanders are intended for temporary subcutaneous
implantation to develop surgical flaps and additional tissue coverage.
- Natrelle®150 double lumen gel/saline breast implants are designed to function
as both tissue expanders and long-term breast implants for one-stage breast
reconstruction or augmentation.
- Natrelle®breast implants and tissue expanders contain no latex or natural rubber
materials.
IMPLANT DESIGN FEATURES
Gel and Gel/Saline Implants
• All Natrelle®textured gel and gel/saline implants have a BIOCELL™ textured
surface engineered with a deep open pore design for firm tissue adherence.
• The INTRASHIEL™ shell features a patented barrier coat between two layers of
silicone elastomer to minimise gel diffusion.
• The Natrelle®150 includes a magnetic resonance imaging (MRI) compatible
self-sealing Mini Remote Injection Site which contains a titanium needle guard
to prevent inadvertent puncture through the base of the injection site.
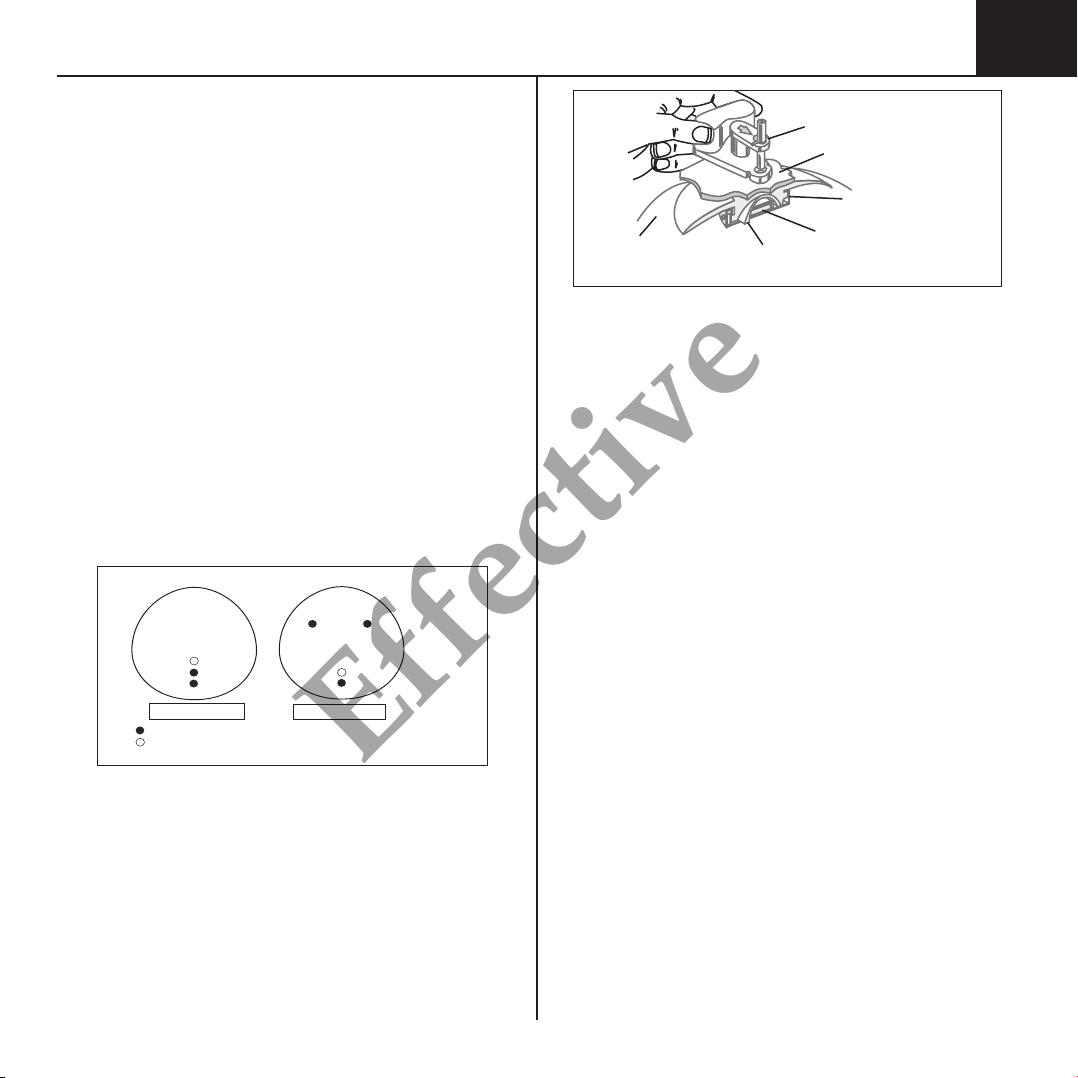
• Anatomically shaped Natrelle®single lumen gel-filled breast implants include
orientation dots to aid in correct implant positioning during surgery (see
Figure 1).
Saline Implants
• Diaphragm valves in Natrelle®saline-filled breast implants are designed for ease
in filling and subsequent air removal.
Tissue Expanders
• BIOCELL™ textured surface is designed to promote tissue adherence.
• The stable base in the Natrelle®133 provides greater control over expansion
direction.
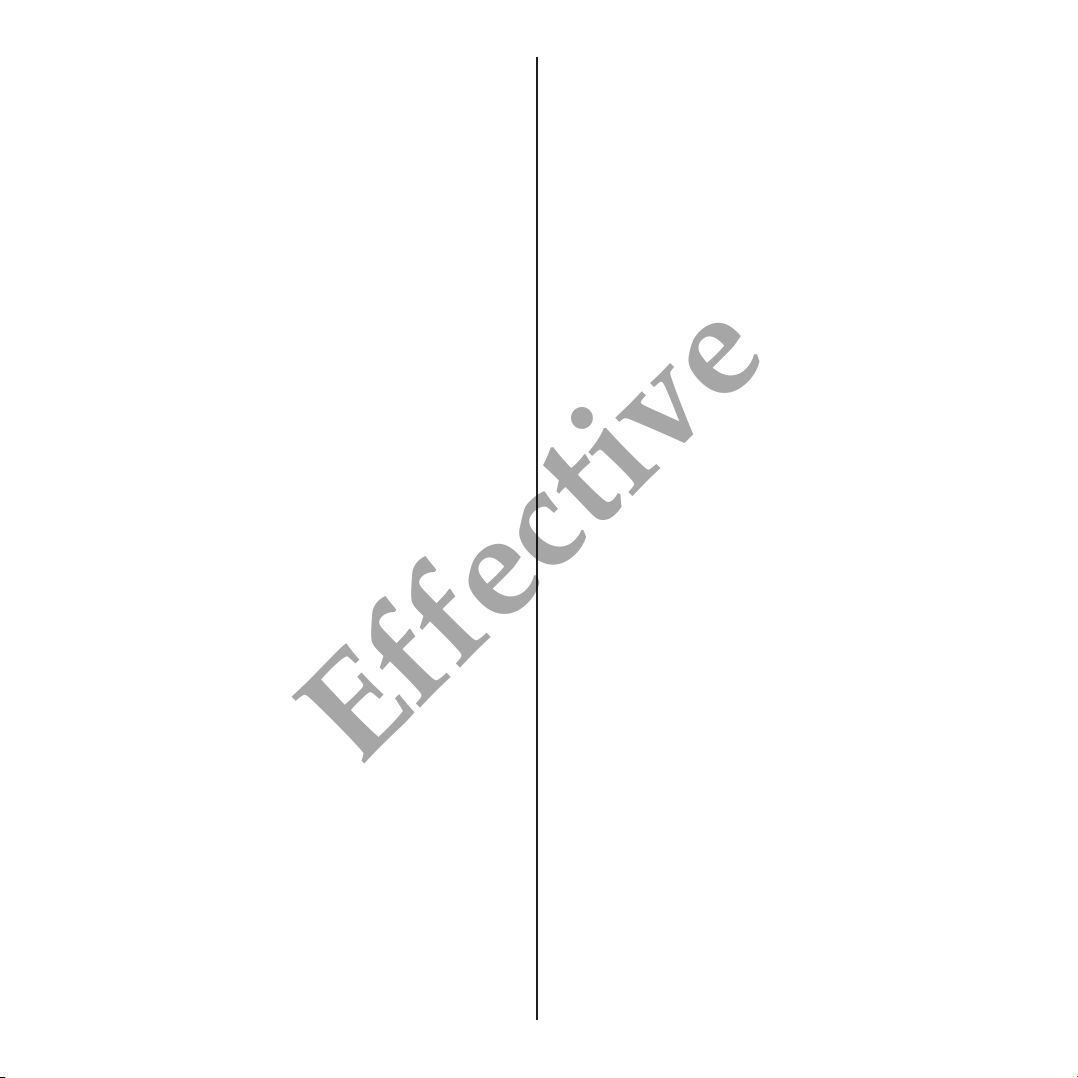
• Natrelle®133 tissue expanders, with integral MAGNA-SITE™ injection site are
supplied with a MAGNA-FINDER™ external locating device. The MAGNA-
SITE™ and MAGNA-FINDER™ contain rare-earth, permanent magnets for an
accurate injection site locating system. In vitro tests show that the MAGNA-
SITE™ is detectable through 60mm of phantom tissue.
• All injection sites are self-sealing and contain a titanium needle guard to prevent
inadvertent puncture through the base of the injection site (see Figure 2).
NATRELLE® ACCESSORIES
• Fill Tube Plug Kits;
• MAGNA-FINDER™
• Other product accessories are available separately.
EACH PATIENT MUST BE INDIVIDUALLY EVALUATED FOR IMPLANT
SURGERY BASED ON THE CLINICAL JUDGEMENT OF A QUALIFIED
SURGEON.
INDICATIONS
• Unilateral or bilateral hypoplasia of the breast.
• Breast reconstruction in patients with adequate tissue covering following
mastectomy or trauma.
• Asymmetry, ptosis, or aplasia of the breast.
• Replacement of implants for medical or cosmetic reasons.
• Congenital deformity of the breast.
• Breast reconstruction in patients following mastectomy or trauma in the case of
tissue expanders.
• Treatment of soft tissue deformities in the case of tissue expanders.
• A patient deemed suitable for breast augmentation must be at least 18 years old
(22 years old in Singapore).
CONTRAINDICATIONS
• Tissue covering determined inadequate or unsuitable by the surgeon.
• Active infection, local and systemic.
• Existing carcinoma of the breast without mastectomy and residual gross local
tumour of the breast after mastectomy.
• Advanced fibrocystic disease considered to be pre-malignant without
mastectomy.
• Use of drugs that might result in high surgical risk and/or significant postoperative
complications, including drugs that would interfere with blood clotting.
• A patient that demonstrates or shows signs of psychological instability (i.e., an
inappropriate attitude or motivation).
• Women who are currently pregnant or breastfeeding.
• Natrelle®133 tissue expanders contain a MAGNA-SITE™ and should not be
used in patients who already have implanted devices that would be affected by a
magnetic field (e.g., pacemakers, drug infusion devices).
• Diagnostic testing with MRI is contraindicated in patients with Natrelle®133
tissue expanders in place. The MRI equipment could cause movement of the
MAGNA-SITE™ tissue expander and result in not only patient discomfort
but also tissue expander displacement, requiring revision surgery. Also,
the MAGNA-SITE™ magnet can interfere with MRI and X-ray detection
capabilities. All other Natrelle®implants are MRI safe.
FIGURE 1
Location of Orientation Dots
Indicates dot location on all sizes.
Indicates additional dot location on selected styles and sizes.
Anterior View Posterior View
FIGURE 2
MAGNA-SITE™ & MAGNA-FINDER™ Locating System
Silicone
Expansion
Envelope Self-Sealing
Silicone
Membrane
MAGNA-FINDER™
Overlying
Expanded Tissue
Puncture-Proof
Titanium Needle Guard
Rare-Earth
Permanent Magnet
NATRELLE®B I T E E
N
Release Date: 29 Sep 2015 00:07:57 GMT -07:00
Expires one day from 21 Dec 2015
Effective
2
RELATIVE CONTRAINDICATIONS/PRECAUTIONS
• Ptotic breasts where nipple falls below the inframammary fold, without
concurrent mastopexy.
• To varying degrees, radiation damage, ulceration, compromised vascularity,
or history of compromised wound healing which may affect tissue covering
suitability.
• Previous repeated contour correction failures.
• Patients about to undergo radiation therapy and/or chemotherapy as this
may make the use of breast implants and tissue expanders more difficult and
increase the risk of complications.
• Physiological condition determined by the surgeon to pose unduly high risk
of surgical and/or postoperative complications. To varying degrees, obesity,
smoking, diabetes, autoimmune disease, coagulopathy, chronic lung or severe
cardiovascular disease may affect patient suitability for surgical implantation.
INFORMATION THAT SHOULD BE PROVIDED TO THE
PATIENT
All patients should be informed of all the potential benefits and risks (see
WARNINGS below) associated with the procedure prior to surgery.
Expected benefits include facilitating emotional healing after cancer, eliminating
external prostheses, regaining body symmetry, allowing freedom in clothing and
physical activities, and improving sexual or interpersonal relationships.
Patients should be informed about the implant options available, the surgical
procedure including implant placement and incision site options. As this surgery
will most likely be carried out under a general anaesthetic patients should be made
aware of the risks associated with anaesthesia. Patients should discuss with their
surgeon any history which may indicate a contraindication (relative or absolute) to
surgery. Post-surgery care should be discussed, including time for wound healing,
the need for any drainage tubes, recuperation duration and the need for implant
integrity to be evaluated on a regular basis after primary surgery. Patients should
be advised to consult a physician or pharmacist before using topical medicines (e.g.
steroids) in the breast area, and if any clinical examination or surgery in the breast
area is planned the patient should inform the doctor or nurse of the presence of an
implant. The surgeon should advise the patient to consult a physician should she
suspect any complications. All patients should receive a patient information book-
let provided by Allergan.
Once the patient has received all the information, she should take at least 30 days
to think about the risks and benefits of having breast implants before making a
final decision.
WARNINGS
The surgeon should advise the patient that management of the complications listed
below may include additional surgery or explantation. Tissue expander patients
should be advised that tissue expanders are only to be used for a short term until
the tissue has expanded sufficiently.
Breast implants have a limited lifetime and the implant may have to be removed
or replaced which may necessitate revision surgery. Various factors, including the
type of implant inserted, the type of surgery, injury to the breast, and excessive
repetitive compression of the implant, may impact the longevity of the implants.
Details on the expected lifetime of the implants are presented in the Rupture/
Deflation section below. As many factors affect the lifetime of a device and are
outside the control of the manufacturer, the life expectancy of the implant cannot
be guaranteed. The surgeon should discuss the necessity of pre-screening mammo-
graphy with each patient as appropriate for her age and medical history.
1. Rupture/Deflation
Gel implants may rupture, and saline or gel/saline implants may deflate at any
time and require replacement or revision surgery. As ruptures are most often
clinically silent, a radiological assessment may be required to aid diagnosis.
Causes of rupture or deflation include:
• Damage by surgical instruments; puncture of the valve may also occur
from improper insertion of the fill tube for saline implants.
• Other trauma during surgery, such as improper handling or manipulation.
• For Natrelle®150 double lumen implants, Natrelle®saline implants and
Natrelle®133 tissue expanders, underfilling below the recommended fill
volume range for the individual implant may result in folds, fold abrasion,
and potentially, crease-fold failure; overfilling above the recommended fill
volume range for the individual implant may compromise shell integrity.
• Capsular contracture, or abrasive calcifications in the fibrous capsule.
• Closed or external capsulotomy.
• Stressors such as trauma, intense physical activity, vigorous massage and/
or manipulation.
• Excessive compression during mammographic imaging.
• Leakage through remote port devices or through an unsealed or damaged
valve.
• Umbilical endoscopic-assisted approach; preliminary reports indicate that
there may be a higher incidence of deflation with this approach.
Long term Allergan Post-Market Surveillance data over fourteen years
on single lumen and double lumen gel/saline breast implants indicates a
rupture rate between 0.37%-1.09%. Allergan US clinical study data on gel
implants indicates a rupture rate between 7.7% -9.7% at 10 years.
Long term Allergan Post-Market Surveillance data over fourteen years on
single lumen saline-filled breast implants and tissue expanders indicates
deflation rates between 0.267%-6.99%. Published results from Allergan US
clinical study data indicated a deflation rate of 10.5% for saline implants at
10 years.
2. Capsular Contracture
Formation of a fibrous tissue capsule around an implanted device is a normal
physiological response. Fibrous capsular contracture remains a common
complication following breast implant surgery and is one of the most common
reasons for reoperation. The cause of capsular contracture is unknown,
however it is most likely multifactorial and may be more common following
infection, haematoma, and seroma. Contracture develops to varying degrees,
unilaterally or bilaterally, and may occur within weeks to years after surgery.
Contracture of the fibrous capsular tissue surrounding the implant may
cause a range of symptoms including firmness, discomfort, pain, distortion,
palpability, and/or displacement. Severe cases are considered the most
clinically significant, and may require surgical intervention. Capsular
contracture may recur subsequent to corrective surgical procedures.
DO NOT treat capsular contracture by external compression or massage,
which may result in implant damage, deflation, folds, and/or haematoma.
3. Infection
Infection around a breast implant may occur within days, weeks, or even
years, after surgery. Signs of acute infection reported in association with
implants include erythema, tenderness, fluid accumulation, pain, and fever.
Erythema may also occur as a normal response to expansion. Infection that
is unresponsive to treatment may require implant removal. Very rarely, Toxic
Shock Syndrome has been reported as a possible complication of breast
implant surgery and may also be associated with other types of implant
surgery.
4. Necrosis
Necrosis may inhibit wound healing and require surgical correction and/or
explantation. Permanent scar deformity may occur as a result of necrosis.
Placement, expansion and pressure of the remote injection site (in the
Natrelle®150) may induce necrosis particularly with unsuitable skin flaps.
Do not use microwave diathermy in patients with breast implants. Microwave
diathermy has been reported to cause tissue necrosis, skin erosion, and
implant extrusion.
Release Date: 29 Sep 2015 00:07:57 GMT -07:00
Expires one day from 21 Dec 2015
Effective
3
5. Haematoma/Seroma
Haematoma/seroma may occur in the postoperative period inhibiting
wound healing, or have delayed onset, either of which may require surgical
correction and/or explantation.
6. Inflammatory Reaction
Studies evaluating the capsules around textured tissue expanders have reported
possible silicone particles within giant cells, indicative of a local (and non-
specific) foreign body reaction, and silicone granuloma formation. Another
study suggests that certain types of capsule cells, including some perceived
as giant cells, may actually be secretory cells that form in response to the
frictional forces of the tissue expander, providing lubrication at the capsule-
expander interface. In case of an inflammatory reaction, the surgeon is advised
to remove the device from the patient’s body and to secure any evidence
on the possible cause of the inflammatory reaction and treat the patient
correspondingly. It is advised not to replace the implant until the inflammatory
reaction has passed completely and its cause has been eliminated.
7. Extrusion
Unstable or compromised tissue covering and/or interruption of wound
healing may result in extrusion of the implant. In case of an extrusion, the
device should be regarded as contaminated and should be removed. It may be
replaced with another device after the wound has sufficiently healed.
8. Wrinkling and Folds
Palpable, or even visible, wrinkles and folds may occur (this may be caused by
underfilling in the case of saline implants). Folds may result in thinning and
erosion of adjacent tissue, and extrusion of the implant. Folds may also result
in crease-fold failure and rupture/deflation of the implant. If wrinkling occurs,
the device may be replaced with an implant with a different filler or shape.
9. Interference with Standard Mammography/Self-Examination
The patient should continue to perform regular breast examinations for
cancer screening; however, this may be more difficult with an implant.
The patient should be informed by the physician about the possible
interference of the implant on the self-examination of the breast.
Patients should be instructed to inform their radiologists of the presence of
an implant. With breast implants, routine screening mammography will be
more difficult as the implant may interfere with diagnostic imaging. Because
the breast and implant are squeezed during mammography, an implant
may rupture during the procedure. More x-ray views may be necessary for
women with breast implants; therefore, a patient may receive more exposure
to radiation. However, the benefit of mammography is likely to outweigh
the risk of the additional x-rays. Ultrasound may be a useful adjunct to
mammography. Breast tissue imaging may be improved by submuscular
placement of the implant. Orientation marks on anatomical gel-filled
implants may be visible on mammographic images.
10. Pain
As expected following any invasive surgical procedure, pain of varying
intensity and duration may occur following implantation. In addition,
improper size, placement, surgical technique, or capsular contracture may
result in pain associated with nerve entrapment or interference with muscle
motion. Unexplained pain must be promptly investigated.
In the case of tissue expanders, the expansion process may cause some
discomfort, but should not cause excessive pain. Pain may indicate expansion
beyond tissue tolerance, which could result in ischemia and necrosis. Further
expansion should be discontinued until the pain is resolved.
11. Breast Feeding and Sensation
Sensation in the nipple and breast can increase or decrease after implant
surgery, is typically lost after complete mastectomy where the nipple itself is
removed, and can be severely lessened by partial mastectomy. Breast implants
may impact the ability to breast feed, though there is no conclusive clinical
study data to support this. The periareolar incision may be associated with a
higher likelihood of breast feeding difficulties than other incision sites. The
risk of temporary or permanent changes in breast sensation resulting from
breast surgery could interfere with the patient’s ability to breast feed. Nerve
traction and compression have been reported in rare cases in association with
tissue expansion. For saline implants, immediate partial deflation should be a
standard precaution if nerve impingement is suspected, and filling should not
resume until the problem is resolved.
12. Dissatisfaction with Cosmetic Results
Scar deformity, hypertrophic scarring, capsular contracture, asymmetry,
displacement, incorrect size, unanticipated contour, palpability, and sloshing
(Natrelle®150 and saline-filled implants), may occur. In some cases, cosmetic
concerns may also lead to medical concerns. Careful surgical planning and
technique can minimise, but not preclude, the risk of such results. Pre-
existing asymmetry may not be entirely correctable. Revision surgery may be
indicated to maintain patient satisfaction but carries additional considerations
and risks. If the patient is dissatisfied with the cosmetic result, revision
surgery may be indicated; the device can be replaced with another device of
different height, width, projection, volume, shape or filling, or may be placed
in a different position in order to achieve a cosmetic result which is more
pleasing to the patient.
Re-positioning of the implant during subsequent procedures should be
carefully evaluated by the medical team and care taken to avoid
contamination of the implant. Use of excessive force during any subsequent
procedure can contribute to localized weakening of the breast implant shell
potentially leading to decreased device performance.
13. Rotation
Rotation of an anatomical implant may occur. Proper placement and pocket
dissection reduces the risk of occurrence. Revision surgery may be necessary
to correct rotation. In case of rotation, it is advised to rotate the device back
into its correct position in an open surgical procedure. Reshaping of the
implant pocket may be necessary to avoid any further rotation in the future.
14. Ptosis
Ptosis occurs naturally in all breasts over time. In case of ptosis, a mastopexy
may be performed and/or the device may be replaced by another product with
a larger volume or greater projection.
15. Distortion
Tissue expansion is a time and labour intensive process that may cause
temporary discomfort and distortion. If distortion occurs, the cause should be
analysed and eliminated.
16. Calcification
Calcification commonly occurs in mature breast tissue with or without
implantation. Microcalcification after implantation typically occurs on or
around the fibrous capsule in thin plaques or accumulations. Extensive
microcalcification may cause breast hardness and discomfort, and may
necessitate surgical intervention.
17. Tissue Atrophy/Chest Wall Deformity
Pressure of a breast implant or expander may cause tissue atrophy. In rare
cases chest wall deformity has also been reported in association with the use
of breast implants and tissue expanders.
18. Gel Diffusion
Minute quantities of silicone may diffuse through the elastomer envelope
of gel-filled implants. The detection of small quantities of silicone in the
periprosthetic capsule, axillary lymph nodes, and other distal regions in
patients with apparently unruptured, conventional gel-filled implants has
been reported in the medical literature. However, there has been only limited
evidence in medical literature associating gel diffusion with local complica-
tions in breast implant patients. If significant gel diffusion occurs, the device
should be checked for any possible leakage or flaws.
Release Date: 29 Sep 2015 00:07:57 GMT -07:00
Expires one day from 21 Dec 2015
Effective

4
19. Adulterated Fill
Do not use adulterated fill. Saline implants and tissue expanders are to
be filled only with sterile saline for injection, and only as described in
Instructions for Use.
20. Inadequate Tissue Flap
Inadequate tissue flap following expansion may occur and may require
additional surgery and expansion.
21. Deformation
The unique nature of the highly cohesive silicone implant may require a
larger incision compared to the incision size required for other silicone-filled
implants to avoid skin edge trauma, implant deformation or separation/
disruption of the gel. Excessive force upon insertion of the implant may
compromise the precisely defined shape of the device, potentially leading to
an undesirable cosmetic outcome.
RESEARCH ON SILICONE IMPLANTS
A report published in 1998 by a US National Science Panel, appointed by Judge Sam
Pointer, evaluated the scientific data on silicone breast implants in relation to connective
tissue diseases and immunologic dysfunction. No association was found between
silicone gel-filled implants and any of the definite connective tissue disorders (including
Sjögren’s Syndrome) or other autoimmune/rheumatic conditions. They found that
women with silicone breast implants do not display a silicone-induced systemic
abnormality in the types or functions of cells of the immune system.
In 1999, an independent review from a committee at the Institute of Medicine in
the US reported that connective tissue disorders, cancer, neurological diseases or
other systemic complaints or conditions are no more common in women with
breast implants than in women without implants. They concluded that a review
of the toxicology studies of silicones and other substances known to be in breast
implants does not provide a basis for health concerns.
Lymphoma, including anaplastic large T-cell lymphoma (ALCL) – Information
from medical literature has suggested a possible association, without evidence
of causation, between breast implants and the very rare occurrence of ALCL
in the breast. The disease is exceptionally rare, may present as a late occurring
peri-prosthetic seroma, and occurs in women with and without breast implants.
Specific testing is needed to distinguish ALCL from breast cancer. The majority of
the reported cases had an indolent clinical course following capsulectomy with or
without adjuvant therapy, which is generally uncharacteristic of systemic ALCL.
Treatment should be determined in consultation with a hemato-oncologist.
INSTRUCTIONS FOR USE
SURGICAL PROCEDURE
Allergan relies on the surgeon to know and follow the proper surgical procedures
with Natrelle®implants. The surgeon must carefully evaluate implant size and
contour, incision placement, pocket dissection and implant placement criteria
with respect to the patient’s anatomy and desired physical outcome. Planning
should include clear delineation of aesthetic goals to ensure mutual understanding
between surgeon and patient. The surgeon should observe current and accepted
techniques to minimise the risk of adverse and potentially disfiguring reactions.
Natrelle®products are designed and tested for compatibility with sterile water and
saline solution. Other substances, such as alcohol or other chemical agents have
not been tested in combination with the Natrelle®products.
Do not immerse the implant in povidone-iodine solution (for example, Betadine®).
If this solution is used in the pocket, ensure that it is rinsed thoroughly so no
residual solution remains in the pocket
Natrelle®products should not be exposed to extreme heat, cold or pressure.
No excessive force should be used while implanting or removing an implant or
expander, and, accordingly, the skin incision should be planned for a sufficient size.
No sharp objects such as knives or needles should be used in direct vicinity of any
Natrelle®product, with the exception of the instruments used to fill an expander or
saline-filled implant via the specially designed valve or port.
Do not alter the implants or attempt to repair or insert a damaged device.
Do not place more than one implant per breast pocket.
Back-up implants must be available during the procedure.
SINGLE USE
These products are intended for single use only.
DO NOT reuse explanted products.
DO NOT attempt to re-inflate saline implants following implantation.
RISKS ASSOCIATED WITH REUSE
Natrelle®breast implants and tissue expanders are not intended to be re-sterilized
or re-used. The cleaning and autoclaving process can cause damage to the breast
implants/tissue expanders, which could lead to loss of structural integrity. Reuse of
the device can cause risk of infection to the patient.
PRODUCT IDENTIFICATION
Product labels are supplied within the internal product packaging of each Natrelle®
implant. The product labels provide specific information which allows product
identification.
Important: These labels must be attached to the patient and hospital/doctors
records to ensure product identification and device traceability.
STERILE PRODUCT
Each implant is sterilised by dry heat sterilisation and is supplied in a sealed,
double primary package.
STORAGE CONDITIONS
Avoid prolonged exposure to extreme storage conditions. Store these devices at
ambient room temperatures and at atmospheric pressure and in dry conditions
away from direct sunlight.
HOW TO OPEN STERILE PRODUCT PACKAGE
Remove the implant and accessories (where applicable) from their packages in an
aseptic environment and using talc-free gloved hands.
DO NOT expose the implant to lint, talc, sponge, towel, skin oils or other surface
contaminants.
1. A non-sterile team member peels open the outer package.
2. The surgeon/scrub nurse removes the inner package and places it into the
sterile field.
3. Peel open the inner package.
4. Gently retrieve the implant.
Prior to use, keep the implant covered in the inner package, to prevent contact with
airborne and surgical field particulate contaminants.
PRELIMINARY PRODUCT EXAMINATION
Prior to use, examine the implant for any evidence of damage or particulate
contamination.
GEL AND GEL/SALINE IMPLANTS
IMPLANT PLACEMENT
Ensure incision is sufficiently large, particularly for textured implants, to facilitate
insertion and avoid damage to the device. Inadequate pocket dissection increases
the risk of rupture and implant malposition. DO NOT use excessive force during
placement of gel-filled implants. Silicone gel may be permanently deformed due to
over-manipulation, resulting in deformation of the shape.
NATRELLE®150 IMPLANT PLACEMENT AND FILLING TECHNIQUES
1. Expandable Breast Implant Placement
Plan and dissect the surgical pockets for placement of the implant and the Mini
Remote Injection Site, using current and accepted surgical techniques. Precise
pocket dissection is recommended, and over-dissection should be avoided.
a) Place the implant flat and correctly oriented in the pocket.
b) Place the Mini Remote Injection Site flat and correctly-oriented in a
separate subcutaneous pocket, ensuring its palpability. The site for the
Release Date: 29 Sep 2015 00:07:57 GMT -07:00
Expires one day from 21 Dec 2015
Effective
Table of contents
Languages:
Other Allergan Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual