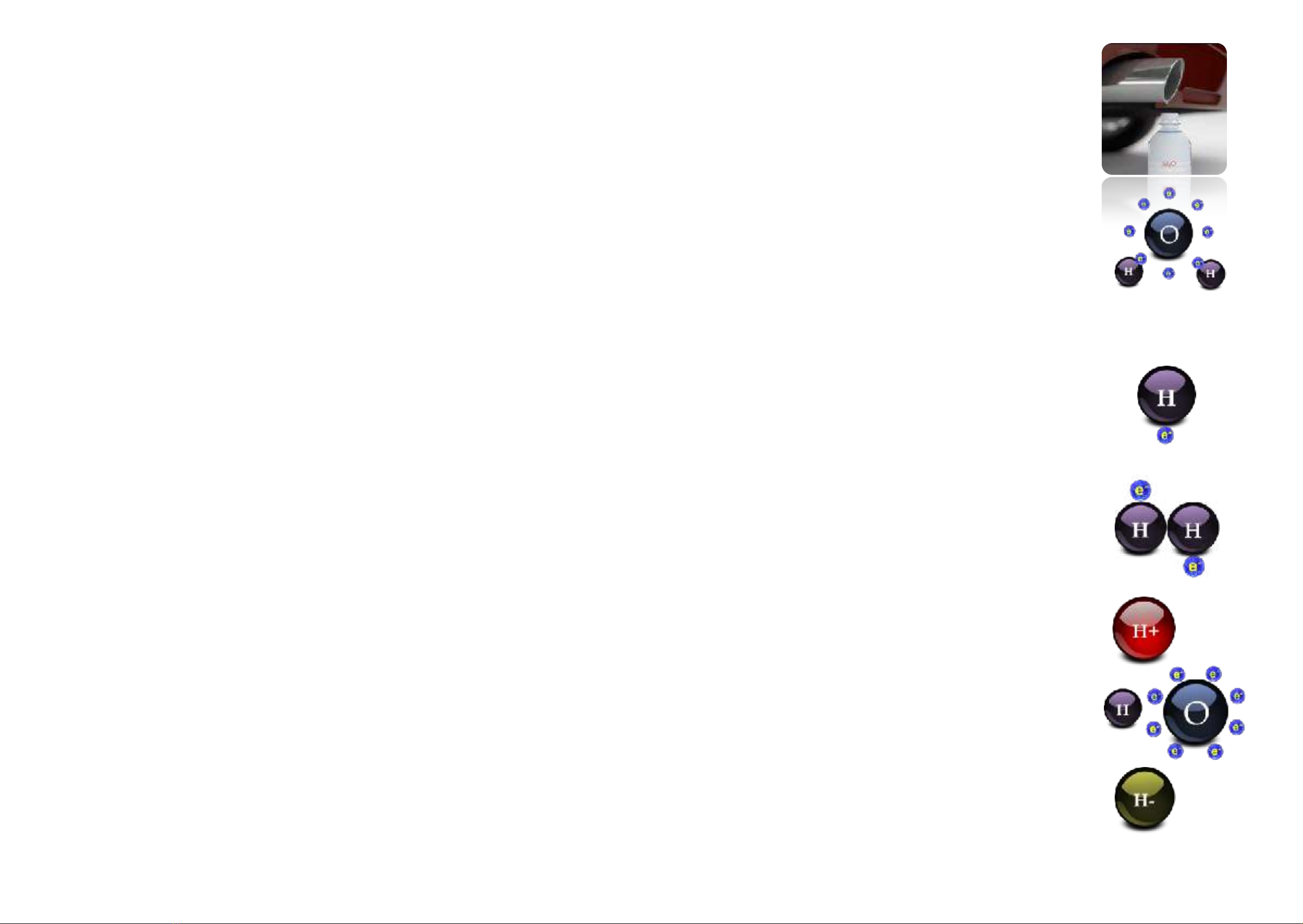3 – Hydrogen – The fundamental concepts
„I run on Hydrogen“. To see Hydrogen cars, out of which no emissions are emitted and only clear water
drips out, is still seldom in our smog-laden cities. Yet there is no doubt that Hydrogen gas presents the
most interesting form of alternative energies of the future. For 1 kg of Hydrogen brings 33,33 kWh/kg
onto the road. Neither petrol (12 kWh/kg) nor natural gas (max. 13,1 kWh/kg) can keep up. Hydrogen,
with the H symbol that stands for Hydrogenium (“The Water Producer”) is the most common element in
the universe. It makes up 75% of the total mass of our solar system. Yet on our planet Earth it is more of a
scarce good. Only 0.12% of the total mass consists of Hydrogen. Most of it is H2O which has bonded as
“energy-less” water in our oceans. Water, H2O, is Hydrogen gas H2which has been combusted by
oxygen. This occurs, for example, with sugar which is converted from food into energy. So Hydrogen
doesn’t only provide energy for fuel cells for cars, but also for the cells in the body. The H Hydrogen
atom is made up of one positively charged nucleus, the proton, which is orbited by a negatively charged
electron. The smallest of all atoms is also called “nascent” Hydrogen: That means “hydrogen in its birth
phase”, for an H-atom does not stay alone for long, it bonds with a second H-atom to make what we
usually call Hydrogen, H2… A further description for this Hydrogen atom is “Hydrogen radical”.
Often Hydrogen gas H2 is confused with the Hydrogen ion H+. This corresponds to an H-atom without an
electron, in short it is a single proton. Positively charged Hydrogen ions are the measure of “acidity”.
They occur by the splitting off of a hydroxide ion (OH-) from water (H2O). If there are more hydroxide ions
in an aqueous solution, it is alkaline, if there are more H+ ions (protons), then it is acidic.
Negatively charged Hydrogen ions (Hydride ions) theoretically also exist. Yet they are so unstable that
they only occur as compounds.