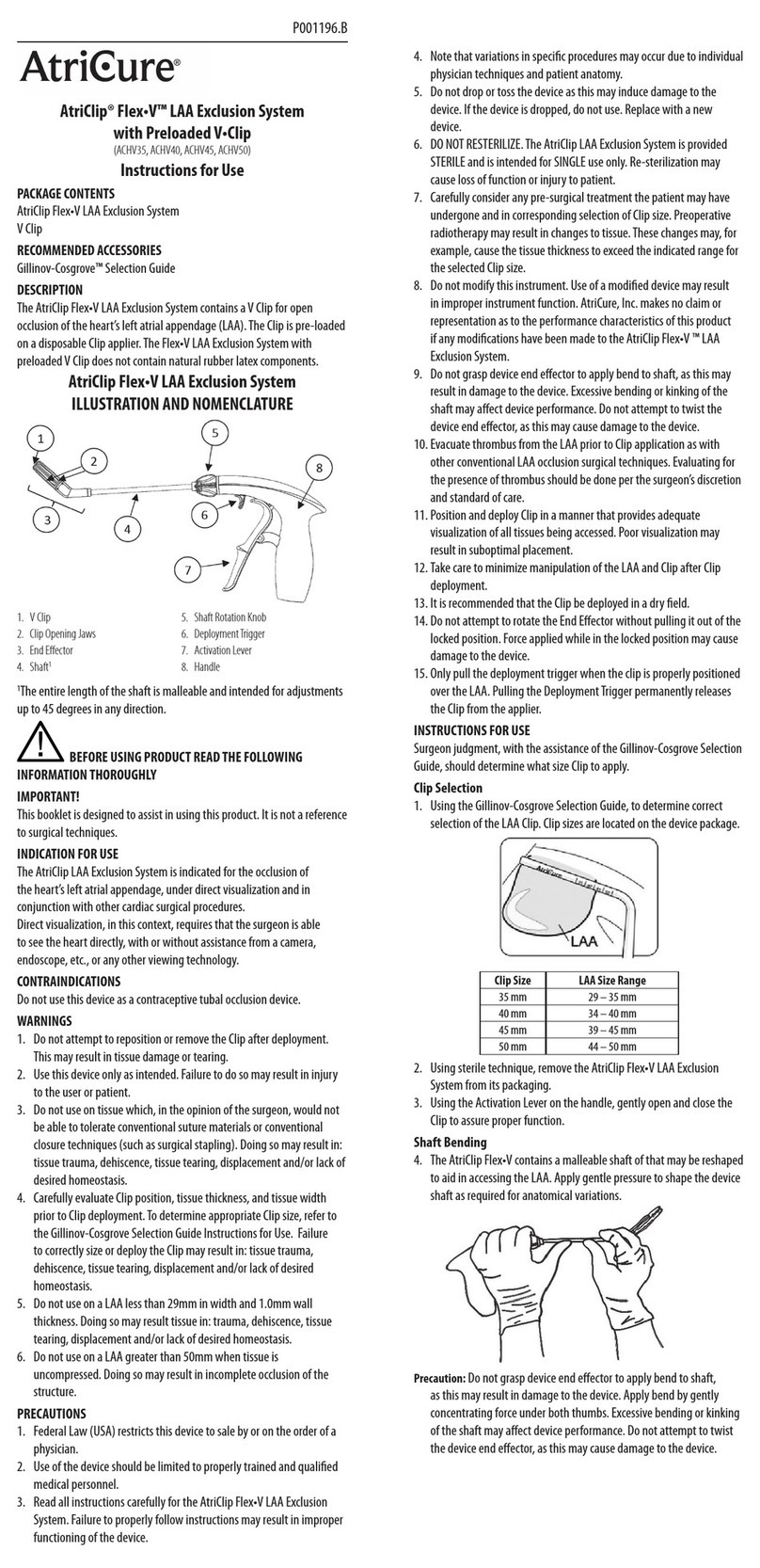
CS-3000 RF Generator Unit Operator Manual
AtriCure, Inc.
IFU-0022.A2021/04
Page 3 / 12
Preface
The nContact Model CS – 3000 Radiofrequency Generator Unit is used to transmit
radiofrequency (RF) energy for localized tissue heating resulting in tissue coagulation.
The unit operates in Power Control and Diagnostic Evaluation modes and is de-
signed specically for use with nContact coagulation devices and accessories.
Indications
For use only with nContact Coagulation Devices, RF Coagulation Cable, and
Sensing Cable.
Contraindications
o The use of the nContact Model CS – 3000 RF Generator Unit, Coagula-
tion Device and accessories is contraindicated when, in the judgment of
the physician, surgical electrocoagulation procedures using RF energy
would be contrary to the best interests of the patient.
o Use in the presence of internal or external pacemakers or internal
cardioverter / debrillators (ICDs) and monitoring equipment may require
special considerations.
Non-Sterile
The nContact Model CS – 3000 Radiofrequency Generator Unit is provided
non-sterile and is not intended to be used within the sterile eld. Do not sterilize the
CS-3000 RF Generator with any sterilization method or the CS-3000 RF Generator
may be damaged. Follow cleaning instruction in chapter 3 to clean CS-3000 RF
Generator.
Warning
o Carefully read all instructions before use.
o Use of radiofrequency energy in patients with internal or external
pacemakers or ICDs and monitoring equipment may require special
consideration. The attending Cardiologist and/or the pacemaker/ICD
manufacturer should be consulted before electrocoagulation surgery.
o Hazardous electrical output. This equipment is for use only by qualied
medical personnel trained in the use of electrocoagulation surgery. Fail-
ure of the high-frequency surgical equipment could result in unintended
increase of output power.
o Electric shock hazard. Do not remove the cover of the nContact RF
Generator Unit Model CS-3000. There are no user-serviceable parts
inside the generator. Refer servicing to qualied personnel only (see
information contained in “Customer Service / Equipment Servicing”).
o Interference produced by the operation of high-frequency surgical equip-
ment may adversely inuence the operation of other electronic medical
equipment such as monitors and imaging systems.
o Never increase Power beyond what is minimally required without rst
inspecting the integrity and contact of the coagulating device.
o Care should be taken to ensure that the device is not in contact with
tissue that is not going to be coagulated (e.g. vascular and nerve tissue),
to avoid inadvertent tissue damage.
o Avoid contact between the Coagulation Device and other surgical
instruments, staples or other objects while coagulating. Inadvertent
contact with objects while coagulating could lead to conduction of RF
energy or heat and unintentional coagulation of tissues in contact with
those objects.
o Burns to the physician’s hands are possible if an RF activated device
electrode comes into contact with a metal instrument or surface.
o The Coagulation Devices and RF Coagulation Cable are provided
sterile and are intended for single patient use only. Do not reprocess or
reuse. Reuse can cause patient injury and the transmission of infectious
disease(s) from one patient to another.
o The coils on the distal end of the Coagulation Device must be kept
clean of coagulum during surgery to avoid loss of power. Do not clean
coagulum o the electrode of the device with an abrasive cleaner or
electrosurgical tip cleaner. The electrodes could be damaged, resulting
in device failure.
o The use and proper placement of an Indierent Electrode is a key
element in the safe and eective use of electrosurgery, particularly in the
prevention of patient burns.
Precautions
o Radiofrequency surgery uses high-frequency energy output. Do not
perform procedures if ammable or explosive media are present.
Non-ammable agents should be used for cleaning and disinfection.
o Make sure the patient is not in contact to earthed metal during the oper-
ation of the CS-3000 RF Generator. Always use appropriate insulation
between the patient and metal surfaces that may connect to earthed
ground. Follow the manufacturer’s directions for the placement of the
indierent, dispersive electrode and for proper insulation between the
patient and any metallic surfaces.
o Maintain safe handling techniques during electrocoagulation due to
electric elds and hot metallic surfaces.
o Do not touch the electrode surface of the Coagulation Device and the
Indierent, Dispersive Electrode at the same time, especially when
operating the Model CS-3000 RF Generator. Supercial skin burns
could occur.
o This equipment has been tested and found to comply with the limits
for medical devices to the IEC 60601-1-2:20151. These limits are
designed to provide reasonable protection against harmful interference.
This equipment generates, uses, and can radiate RF energy and, if
not installed and used in accordance with the instructions, may cause
harmful interference to other devices in the vicinity. However, there is no
guarantee that interference will not occur. If this equipment does cause
harmful interference to other devices, which can be determined by
turning the equipment o and on, the operator is encouraged to correct
the interference by:
• Relocating or moving the equipment
• Increase the separation distance between the equipment
• Connect the equipment into dierent outlets
• Consult AtriCure, Inc. representatives for help
o The Coagulation Device, RF Generator, Cables and Accessories have
been tested as a system. Use of another manufacturer’s accessories
may cause damage to the equipment or injury to the patient.
o The use of accessory equipment not listed in this operator’s manual as
complying with the equivalent safety requirements of this CS-3000 RF
Generator may lead to a reduced level of safety. Accessory equipment
connected to the CS-3000 RF Generator must be in compliance with
IEC-60601-1 requirements. Anyone who connects additional equipment
to the CS-3000 RF Generator is responsible for compliance with the
requirements of industry standard IEC 60601-1-1. If in doubt, consult
the technical service at AtriCure, Inc.
o While the distal portion of the Coagulation Device is designed to be
malleable to conform to the anatomy of the area to be coagulated,
excessive or rough shaping of the device may damage its internal
components. Care should be taken when handling the distal end of the
device near the electrode with surgical instruments – do not squeeze or
clamp the electrode.
o Inspect the Coagulation Device, RF Coagulation Cable, and packaging
before use. If any breach of the packaging is found, the sterility of the
product cannot be guaranteed, and the product should not be used.
o Ensure complete separation of the Indierent, Dispersive Electrode
and EKG electrodes to prevent interference with patient monitoring
equipment. Needle monitoring electrodes are not recommended. Mon-
itoring systems incorporating high frequency current-limiting devices are
recommended.
Guidance and Manufacturer’s Declaration – Electromagnetic Emissions
The Model CS-3000 RF Generator is intended for use in the electromagnetic
environment specied below. The user should assure that the CS-3000 is used
in such an environment
Emissions Test Compliance Electromagnetic Environment -
Guidance
RF Emissions
CISPR 11 Group 1
The CS-3000 RF Generator intentionally
transmits RF energy as its intended
function. Nearby electronic equipment
may be aected.
RF Emissions
CISPR 11 Class A
The CS-3000 RF Generator is suitable
for use in all establishments other than
domestic and those directly connected
to public low-voltage power supply
network that supplies buildings used for
domestic purposes.
Harmonic Emissions
IEC 61000-3-2 Class A
Voltage Fluctuations/
Flicker Emissions
IEC 61000-3-3
Complies
Classication in accordance with EN 60601-1
Safety Met Labs Mark Information
CLASS 8750 01 – MEDICAL ELECTRICAL EQUIPMENT/SYSTEMS
CLASS 8750 81 – MEDICAL ELECTRICAL EQUIPMENT/SYSTEMS –
Certied to US Standards
Radio Frequency Ablation Device, Model nContact CS-3000, rated: 100-240V~
50-60Hz 250VA
1. Type of protection against electric shock: Class 1
2. Degree of protection against electric shock: Type CF
3. Degree of protection against ingress of water: IPX1
4. Equipment not suitable for use in presence of a ammable anesthetic
mixture with air or with oxygen or nitrous oxide
5. Mode of operation: Intermittent
Environmental Conditions: Normal: 10-40°C, 30-75% rH. 700-1050mb