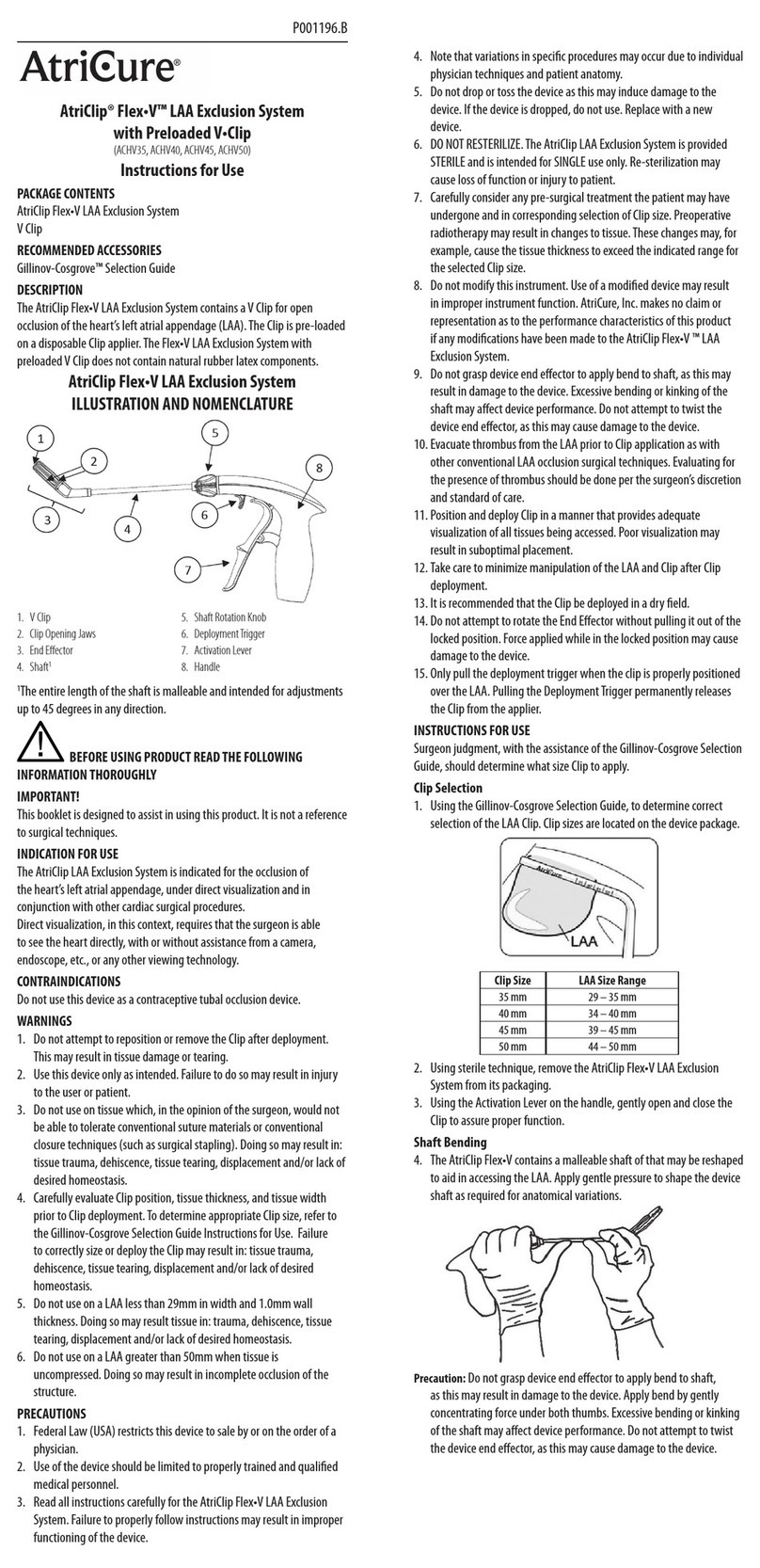
6
INDICATION FOR USE
The ATRICURE Bipolar (Transpolar)
System is intended to ablate soft tissue
during General surgical procedures.
CONTRAINDICATIONS
The Bipolar (Transpolar) System is not
indicated for contraceptive coagulation
of the fallopian tubes.
Potential Complications
Device
Possible complications related to the
creation of the linear lesions in cardiac
tissue using a clamp-type device maybe
be included but not limited to:
•Tissue Cutting
•Perioperative heart rhythm
disturbance (atrial and/or
ventricular)
•Postoperative embolic
complications
•Pericardial effusion or
tamponade
•Injury to the great vessels
•Valve leaflet damage
•Conduction disturbances
(SA/AV node)
•Acute ischemic myocardial
event
•Injury to unintended
surrounding tissue structures,
including tears and punctures.
•Bleeding requiring intervention
to repair.
•Extension of cardiopulmonary
bypass
Procedure
Serious adverse events that may be
associated with surgical ablation
procedures on the heart (stand alone or
concomitant to other cardiac surgery),
include:
•Death
•Excessive bleeding related to the
procedure (defined as bleeding
which requires >3 units of blood
products and/or surgical
intervention),
•Cardiac tamponade (if either open or
catheter drainage is required),
•Pulmonary vein stenosis,
•Restrictive (constrictive) pericarditis,
•Endocarditis,
•Myocardial infarction (MI) per ACC
guidelines,
•Stroke (resulting in permanent
neurological deficit),
•Transient Ischemic Attack (TIA),
•Thromboembolism
•Diaphragmatic paralysis,
•Esophageal-LA fistula or
esophageal rupture,
•Atrial perforation or rupture,
•Ventricular perforation or rupture,
•Atelectasis,
•Pneumonia,
•Congestive Heart Failure,
•Cardiac Valve Injury,
•Persistent Pneumothorax (requiring
intervention),
•Excessive Pain and Discomfort,
•Deep Sternal Wound Infection,
•Ventricular Arrhythmia (V.
Tachycardia or V. Fibrillation)
•New Sinus Node Dysfunction, and
•Drug Reaction
WARNINGS
•Do not touch the electrodes of the
ISOLATOR while activating the
ASU. Touching the ISOLATOR
electrodes during ASU activation
could result in an electrical shock or
burn to the operator.
•Do not touch the electrodes of the
ISOLATOR to metal staples or
clips, or to sutures while activating
the ASU. This may damage the
ISOLATOR or tissue, or result in an
incomplete ablation.
•Do not use abrasive cleaners or
electrosurgical tip cleaners to clean
debris from the Jaws. Use of
abrasive cleaners or electrosurgical
tip cleaners can damage the
electrodes and result in device
failure. Use saline-soaked gauze to
clean debris off the electrodes.
•Do not immerse any part of the
ISOLATOR in liquids as this may
damage the device.