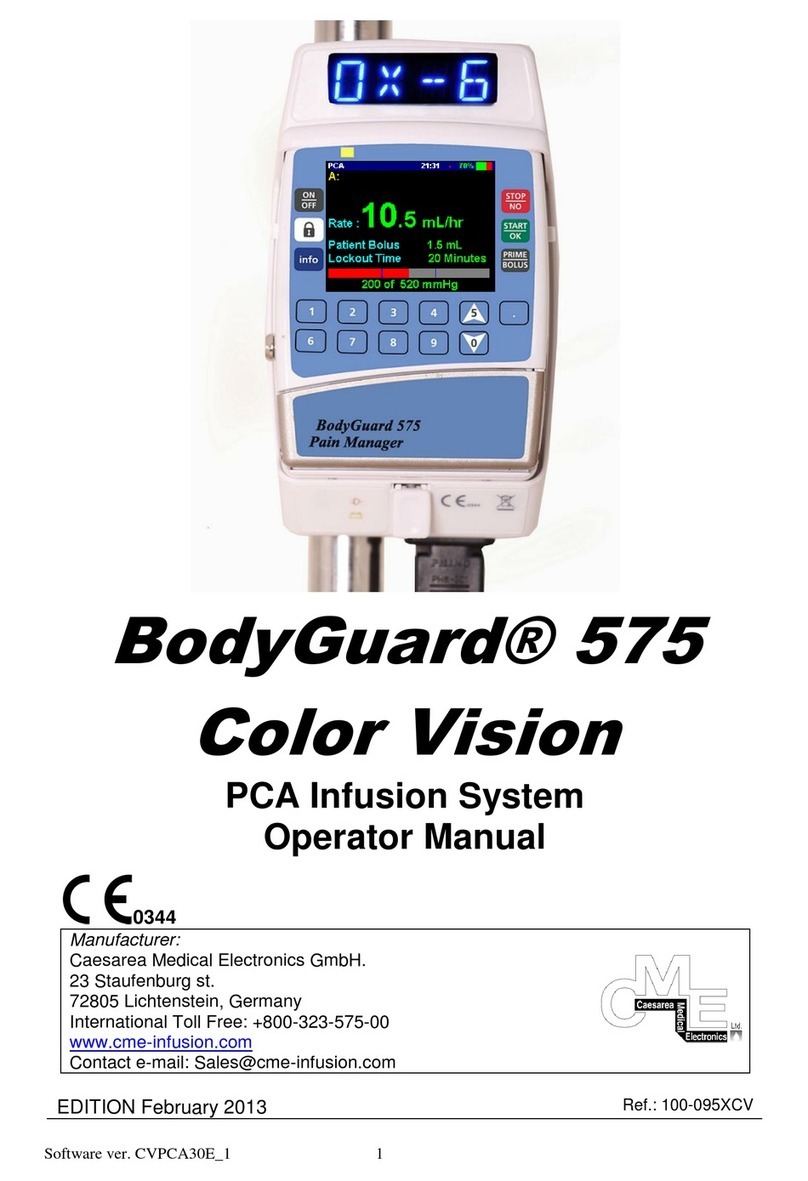
BodyGuard 595 Color Vision™Directions For Use
System Safety Checks
The following details outline the safety checks designed into the BodyGuard 595 Color
Vision™ pump to minimize the possibility of under or over infusions.
Free Flow Protection
The system’s customized IV lines are equipped with a check valve that prevents
free flow towards the patient when the IV line is not attached to the pump. When
the pump is attached to the IV line and delivering fluid, the pressure delivered by
the pump opens the valve. The valve is also one way valve preventing fluids flow
from the patient to the administration line
Post Occlusion Bolus Reduction System (POBRS)
The POBRS function is designed to reduce the bolus that may occur upon the
release of an occlusion following a downstream occlusion alarm. Upon the
detection of a downstream occlusion, the alarm is activated and the pump returns
the IV line pressure to neutral within 15 seconds. Neutral line pressure is achieved
by the reverse operation of the pumping mechanism, and measurement of the IV
line pressure through the in-line pressure detection system.
Unintended Bolus
250mmHg (minimum)
1500mmHg (maximum)
An occlusion may pressurize the infusion tubing, which can result in an unintended
bolus of drug when the occlusion is cleared. In order to prevent this additional
bolus, disconnect the tubing or relieve the excess pressure through a stopcock, if
present. The clinician should weigh the relative risks of disconnection with the risks
of an unintended bolus of drug.
Air-in-Line Detection
BodyGuard 595 Color Vision™ uses two modalities to detect air-in-line. The ultrasonic
detector can be configured between 0.04 ml -1.0 ml on the BodyGuard 595 Color Vision™
pump for single bubble detection while a cumulative check triggers the alarm if an
accumulation of smaller bubbles totals 1.0ml (non-configurable) in any 15 minute period.
Although a single bubble may not exceed the user defined threshold (e.g. 0.5ml) if the
cumulative volume of smaller bubbles exceeds 1ml (e.g. if three 0.4ml bubbles pass the
sensor within a 15 minute period) an ‘Air or Up Occlusion’ alarm is activated. This
accumulation feature is particularly useful when infusing products that create a significant
number of small air bubbles (out-gas) to a patient who is highly sensitive to air (i.e. infants,
neonates, children).
Program Limits (including MediGuardTM toxicity settings)
Under ‘Change Set Up’ users can choose from a number of options to limit protocol
parameters and set safe ceilings on drugs infused. When the MediGuardTM feature is on
users are asked to set the patients weight (kg) and a toxicity ceiling in ml, mg or mcg per
hour per kilogram bodyweight. If users try to set a protocol where the component elements
(basal rate and boluses) exceeds the MediGuardTM limit the pump will alert the user to this
and request they re-confirm their intentions, amend or revise the toxicity ceiling. Level
One user will not be able to change the protocol or ceiling under Select Protocol and will