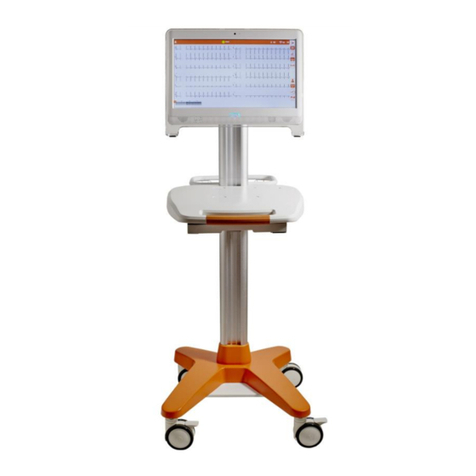
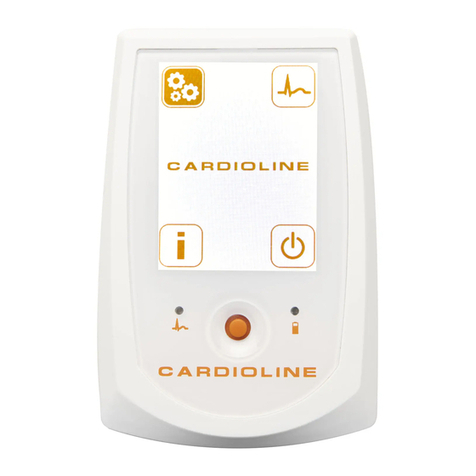
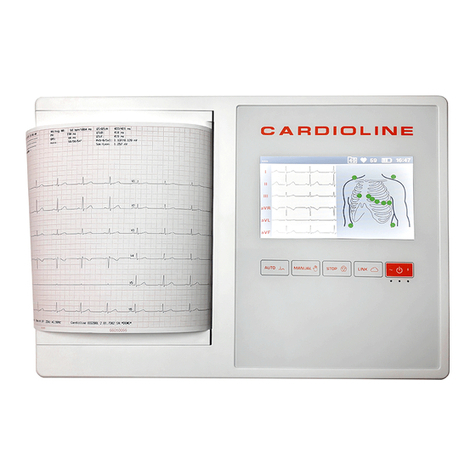
7
1.2 Information and recommendations related to
safe use
- Always use the device according to the instructions in this manual.
- The device is equipped with a set of standard accessories. For reasons
of safety, reliability and conformity with the Medical Devices Directive
93/42/EEC, use only original accessories or accessories approved by the
manufacturer.
- The device is equipped with a special long-life thermal print head writing
system, which allows maximum writing precision. To avoid frequent and
costly replacements and repairs, always use the original paper or paper
approved by the manufacturer. The manufacturer does not accept
responsibility for any damage to the device or any other effect on it,
caused by the use of unsuitable paper.
- Do not subject the device to impact or excessive vibrations.
- Do not allow liquids to penetrate inside the device. If this should
accidentally occur, have the functional efficiency of the device tested by
a Qualified Assistance Centre before using it again.
- Make sure that the value of the supply voltage corresponds to that
indicated on the data plate of the device.
- If you are using the device in connection with others, ensure that: all
connections are made by skilled persons; all connections comply with
safety regulations; all other devices connected respond likewise to
regulations. Non-compliance with regulations can cause physical harm to
the patient connected and to the person operating the device. Should it
be difficult to obtain the necessary information for assessing the risk of
the individual connections, apply directly to the manufacturers
concerned or avoid making the connections.
- If other equipment is used, connected directly or indirectly to the
patient, check the possible risks caused by the sum of the leakage
currents on the body of the patient.
- The device is protected against defibrillation discharges according to
standards IEC 601-1-25; to ensure that the signal is restored, use only
original electrodes or electrodes responding to IEC and AAMI standards.
- If an electrosurgical scalpel is in use, the patient cable should be
disconnected from the device.
- At all events, when defibrillators or high-frequency surgical devices are
being used at the same time, it is essential to take the greatest care. If
there is any doubt concerning their use, disconnect the ECG patient
cable temporarily.
- The device recognizes the impulses generated by a pacemaker and does
not interfere with its operation, as prescribed by standards in use at the
time of drafting this manual.
- Periodically check the efficiency of all accessories and of the device
itself. Use the built-in test function to make a first check of the
equipment's efficiency. Contact the Authorized Technical Assistance
Center whenever the equipment appears to be malfunctioning.