ENGLISH • 9
Section 10: Troubleshooting
Although service and repair of the Cavitron®SPS™Ultrasonic Scaler
should be performed by DENTSPLY®personnel, the following
are some basic trouble shooting procedures that will help avoid
unnecessary service calls. Generally, check all lines and connections to
and from the System, a loose plug or connection will often create
problems. Check the settings on the System’s knobs.
10.1 TroubleshootingGuide
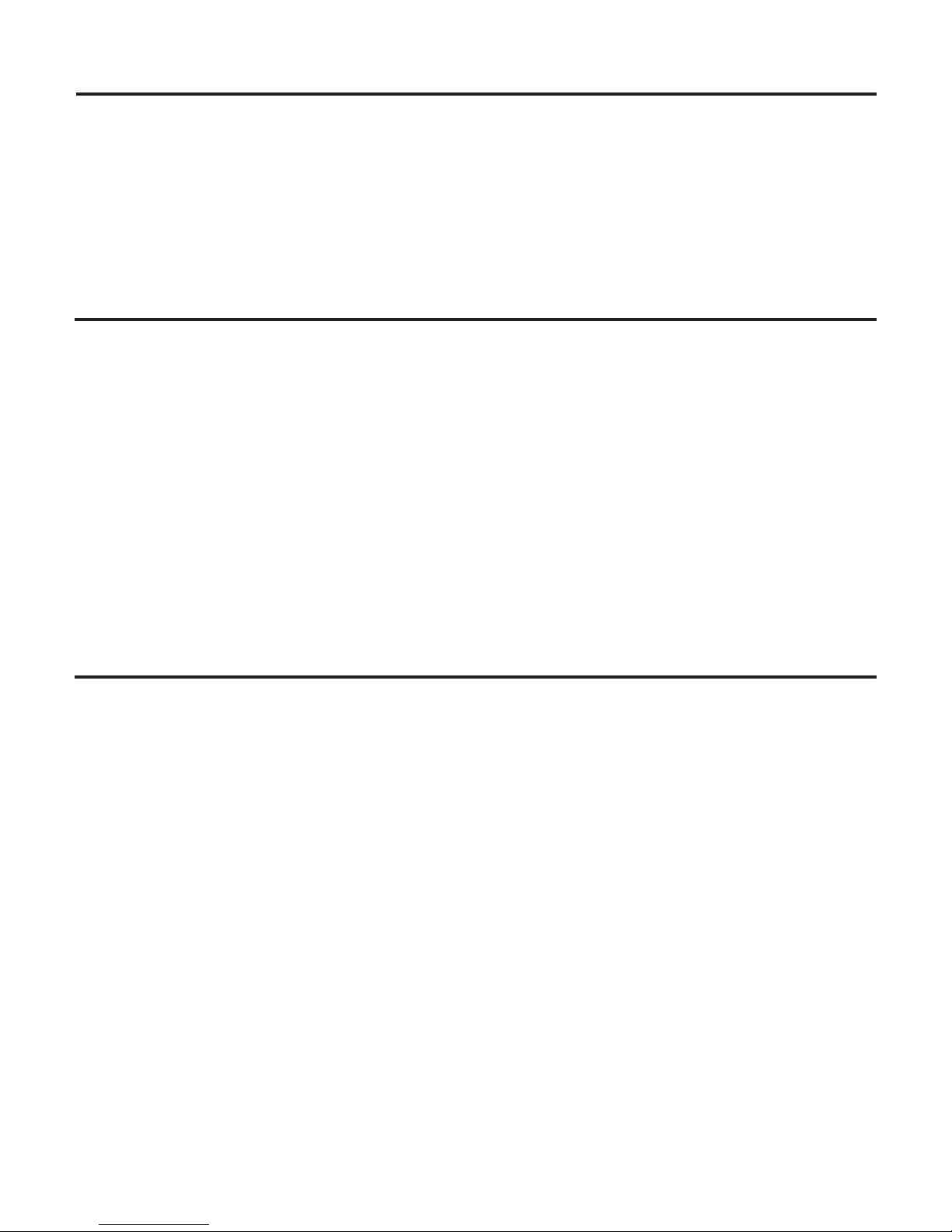
System will not operate:
(Power Indicator Light is not lighted.)
1. Check that the Power switch is in the ON position,
and that the detachable Power Cord is fully seated in
the receptacle on back of System.
2. Check that the System’s three-prong plug is fully
seated in an appropriate AC receptacle, and that AC
current is present.
Power Indicator Light is lighted.)
Section 9: System Care cont'd
a sink or drain. Activate the Foot Control and flush the water
line for at least 2 minutes.
6. Place a sterilized insert into the Handpiece and set the Lavage
Control knob to your preferred operating position.
Between Patients
1. Remove all ultrasonic inserts and handpiece used, clean and
sterilize.
2. Clean and disinfect the surfaces of the cabinet, Power Cord,
Handpiece Connector and cable assembly, Foot Control and
cable assembly by applying an approved non-immersion type
disinfectant solution* carefully following the instructions
provided by the disinfectant solution manufacturer. To clean
system, generously spray disinfectant solution on a clean towel
and wipe all surfaces. Discard used towel. To disinfect system,
generously spray disinfectant on a clean towel and wipe all
surfaces. Allow disinfectant solution to air dry.
3. Connect a freshly sterilized handpiece to its matching cable
connector. Hold the handpiece over a sink or drain and flush
the water line of the unit as above at maximum water flow
for 30 seconds.
4. Place a freshly sterilized insert into the handpiece.
Shut-Down Procedures at the end of the day:
1. Remove all ultrasonic inserts and handpiece used, clean and
sterilize.
2. Turn the System OFF.
3. Clean and disinfect the surfaces of the cabinet, Power Cord,
Handpiece Connector and cable assembly, Foot Control and
cable assembly by applying an approved non-immersion type
disinfectant solution* carefully following the instructions
provided by the disinfectant solution manufacturer. To clean
system, generously spray disinfectant solution on a clean towel
and wipe all surfaces. Discard used towel. To disinfect system,
generously spray disinfectant on a clean towel and wipe all
surfaces. Allow disinfectant solution to air dry.
4. Close the manual shut-off valve on the dental water supply
system.
*NOTE: Water-based disinfection solutions are preferred. Some
alcohol-based disinfectant solutions may be harmful and may
discolor plastic materials.
9.2 WeeklyMaintenance
End of Week Procedures (when connected to a DualSelect™
Dispensing System)
Follow the end of week procedures listed in the DualSelect™
manual.
9.3 Water Filter Maintenance
When the water filter becomes discolored, the filter should be
replaced to prevent reduced water flow to the Cavitron®SPS™
Ultrasonic Scaler. A 10-pack of replacement filters is available by
ordering Part Number 90158 from your local DENTSPLY®
distributor.
1. Disconnect the water supply hose from the water source. If a
quick-disconnect connector is attached to the end of the hose,
relieve the water pressure by pressing the tip of the connector
in an appropriate container and drain the water.
2. Grasp the fittings on either side of the filter disk and twist
counterclockwise. Remove the filter section from either side
of the water hose.
3. Install the replacement filter onto the water hose fittings. The
filter should be positioned to match up with the correct hose
fitting.
4. Hand tighten the two hose fittings in a clockwise direction.
Reconnect the water supply hose, operate the unit to bleed
the air and test for leaks.
1. Check that the Foot Control Connector is fully seated
in the Foot Control Receptacle on the back of the
System.
System operates:
(No lavage flow at insert tip.)
1. Assure that Lavage control is properly adjusted.
2. Check that irrigant supply control valve/s (dental
office water supply) are open.
3. If connected to DualSelect™Dispensing System, check
that fluid level in thse selected bottle is sufficient. Make
sure valves are open when using external water source.
10.2 Technical Support and Repairs
For technical support and assistance call 1-800-989-8826 Monday
through Friday, 8:00 AM to 5:00 PM (Eastern Time). For other
areas, contact your local DENTSPLY®representative.