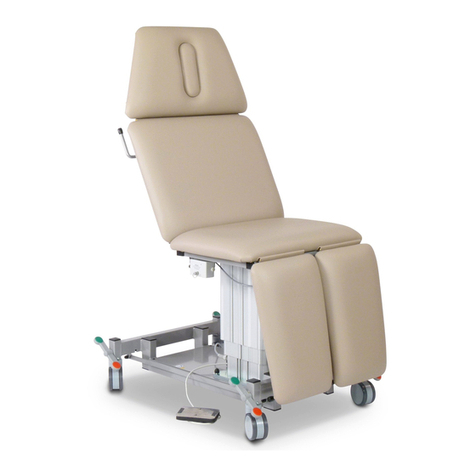
Dewert DX1 Series User manual

Dewert User Manual DX1 Series–Updated 05.2021/1 MDR Page 1 of 16
User Manual
DX1 series model
Dear customer,
Congratulations on purchasing a product of outstanding quality. Use of the best materials from renowned
suppliers guarantees years of trouble-free operation, provided the device is handled correctly and as intended
in accordance with the conditions described in the user manual.
In the unlikely event you need to make a claim, please contact us.
We welcome suggestions from the users of our products.
K.H. DEWERT GmbH
Vollmestr. 7
33649 Bielefeld
Germany
Tel. +49 / 521 400 27-0
Fax +49 / 521 400 27-27
www.khdewert.de

Dewert User Manual DX1 Series–Updated 05.2021/1 MDR Page 2 of 16
Contents
1. Safety information.......................................................................................................................................3
1.1 Applied symbols.................................................................................................................................... 3
1.2 Applied standards.................................................................................................................................3
1.3 Safety regulations.................................................................................................................................3
1.4 Intended purpose..................................................................................................................................3
1.5 Information on setup and use...............................................................................................................4
1.6 Commissioning..................................................................................................................................... 4
1.7 Safety instructions ................................................................................................................................ 4
1.8 Model designation and type labelling ...................................................................................................5
1.9 Meaning of the serial number............................................................................................................... 5
2. Operating manual........................................................................................................................................6
2.1 Table design.........................................................................................................................................6
2.2 Height adjustment.................................................................................................................................6
2.3 Upholstery adjustment..........................................................................................................................6
2.4 Movability (model dependent or optional):............................................................................................7
2.5 Additional equipment............................................................................................................................7
3. Additional accessories (for user-specific table configuration, model dependent)....................................... 8
4. Technical data............................................................................................................................................. 9
4.1 DX1 series............................................................................................................................................9
4.2 Technical data for electric motor ........................................................................................................10
4.3 Technical data for hydraulic system...................................................................................................10
5. Cleaning instructions................................................................................................................................. 10
6. Maintenance and technical inspection......................................................................................................11
7. Safety devices...........................................................................................................................................12
8. Reporting obligation..................................................................................................................................13
9. Disposal ....................................................................................................................................................13
10. Declaration of Conformity .......................................................................................................................14

Dewert User Manual DX1 Series–Updated 05.2021/1 MDR Page 3 of 16
1. Safety information
1.1 Applied symbols
Safety instructions and key sections in these operating instructions are marked with the exclamation mark
symbol on the left. Please pay particular attention to these instructions and sections.
Other symbols possibly used on the device:
Observe instructions for use:
Risk of pinching when adjusting:
Risk of hazard zone:
1.2 Applied standards
This device has been designed and manufactured in accordance with national and international
regulations. This ensures a very high level of equipment safety.
The models described here comply with the following regulations and directives:
Regulation (EU) 2017/745
DIN EN IEC 60601-1
DIN EN ISO 14971
DIN EN 60601-2-52 partly based on
DIN EN IEC 62353
DIN EN ISO 10993-5/-10
DGUV Regulation 3
This device is a Class 1 medical device according to Regulation (EU) 2017/745 (MDR).
1.3 Safety regulations
This section contains a summary of the most important safety information.
Correct operation of the device is essential for safe operation. Therefore, please familiarise yourself with the
contents of these instructions for use before using the device. We recommend that you keep these
instructions for use near the device for future reference. The device may only be used by authorised,
instructed and competent persons who are sufficiently familiar with its adjustment mechanism or have read
and understood the operating manual fully. The manufacturer cannot be held responsible for damage caused
by or involving unauthorised persons.
The user must ensure that the device is not accessible to unauthorised persons or cannot be operated by
unauthorised persons even when left unattended. When leaving the device, it should be secured in such a
way that unauthorised adjustment is impossible.
Keep a sufficient safety distance to the device during all adjustment procedures. Special attention
must be paid to the arms, hands, legs and feet of the user and the patient - RISK OF CRUSHING!
Make sure that there are no objects located directly around or underneath the device!
1.4 Intended purpose
The table is used for the ideal positioning of patients for the purpose of curative and disease treatment,
examination, massage and health therapy.
Table operation and patient positioning on the table may only be performed by professionally trained persons
who have been instructed in its use or who, through experience with other similar medical devices, have
knowledge of its proper use, taking into account possible hazards.
Dewert User Manual DX1 Series–Updated 05.2021/1 MDR Page 4 of 16
Equipped with the options of movability (not wheel-lifting/fixing mechanism), side guards and push handle, the
intended purpose of the table is extended and also provides for the ideal positioning of patients for the
purpose of transport to pre-treatment or post-treatment locations. Patient positioning during the recovery
phase after a medical procedure is also permitted under supervision.
Otherwise, the device must only be moved within the room for cleaning or patient access.
This device has been developed exclusively for use indoors and in normal ambient conditions and can be
used in the following areas: laboratories, medical practices, examination and treatment rooms, hospitals,
clinics, physiotherapy practices, occupational therapy centres and doctors’ surgeries. This table is not classed
as surgical furniture and must, therefore, not be used for surgical purposes. The expected service life is 10
years or 100,000 drive cycles (double stroke = 1x up and down).
1.5 Information on setup and use
When packed, the device may be exposed to the following environmental conditions for approx. 3 months:
Transport and storage temperature: -20° to + 50°
Operating temperature: +10° to +40°
Relative humidity: 30% to 75%
Air pressure: 800hPa to 1060hPa
When transporting the device in a vehicle, it must be secured properly against moving. To do so, lock the
castors (optional equipment) and ensure further safety measures.
When transporting the table, take hold of the underframe, NOT the upper frame.
When setting up the device, do not lift it at the head part, as this may damage the head part and/or the
release mechanism. Hold and lift the table on the left and right-hand side of the frame.
The table must stand securely on its feet or castors on a level, flat and solid surface. Before use, activate the
brakes on the castors and make sure they are working properly.
1.6 Commissioning
The device is ready for use upon delivery.
Remove the power cable from the film packaging on the underframe of the table and connect it properly to a
permanently installed mains socket. When routing the power cable, make sure the cable cannot be crushed,
rolled over or otherwise damaged.
Lock the castors (optional) or the wheel system (optional).
As the operator, carry out a thorough and precise function check once the device has been set up. Prior to
commissioning, clean the device and remove any contamination from transport. Make sure that no connecting
cables from the hand or foot switch to the motor are trapped in the mechanism and thus damaged. Operation
in potentially explosive atmospheres is not permitted.
1.7 Safety instructions
This table may only be used for its intended purpose. Any other use is strictly prohibited and possibly
dangerous. The manufacturer cannot be held responsible for damage caused by improper use. Patients may
only be positioned in preparation for treatment/examination by professionally trained persons.
Please note: This table is not classed as surgical furniture.
Prior to and when adjusting the height of the table, make sure that no persons or objects are located in the
adjustment range of the table and that nobody has their hands on the underframe.
The following basically applies: Never reach into or under the frame of the table when adjusting the height.
Height adjustment can result in injury if the user does not pay due care and attention. Therefore, take great
care when performing this procedure.
When adjusting the upholstery parts, make sure that no persons or objects are in the adjustment range. Make
sure that no persons reach under the upholstery part or lean on the underframe.
Important for the user: When adjusting the upholstery parts, do not reach under the spacers located beneath
the upholstery parts.

Dewert User Manual DX1 Series–Updated 05.2021/1 MDR Page 5 of 16
Always use both hands when adjusting the lying surface elements: Use one hand to operate the adjustment
mechanism and the other hand for the lying surface adjustment.
The lying surfaces and the underframe are not anti-static as standard. Our products are not intended for use
in wet rooms and must under no circumstances be cleaned using so-called bed washers. This would
irreparably destroy the product.
The head part and armrests are only intended to support the patient and must not be used for sitting.
If the underframe is designed with movability (optional), all the castors must always be locked before using
the table.
Do not put a damaged device into operation.
Disconnect the device from the mains (power supply) in the event of a fault or during
maintenance work. To disconnect, be sure to grasp the plug, not the power cable.
This device must not be modified without the express permission of the manufacturer.
When transporting the table, only take hold of the underframe, NOT the upper frame.
1.8 Model designation and type labelling
The exact model designation depends on the choice of frame colour:
-00 white powder coated (RAL 9010);
-03 white aluminium powder coated (RAL 9006);
-04 grey aluminium powder coated (RAL 9007)
and the type of selected height adjustment:
E = electromotive;
/H = hydraulic
The type plate is attached to the underframe on one long side of the table. It provides information about key
table data.
The following symbols are listed there (by way of example), their meanings are:
Read the operating
manual
Serial no.
Applied part Type B
Caution, potential
hazard
Max. load
capacity
CE mark
Date of manufacture
Product may only
be used in dry
rooms
Do not dispose of with
household waste
Address of
manufacturer
Protective
insulation,
protection class II
1.9 Meaning of the serial number
The serial no. is located on the type plate or shown separately next to the type plate of the table. This number
is unique and firmly linked to this specific individual product. It enables us to identify this table model and
trace back assemblies/safety-relevant components at any time. Please always state this serial number when
enquiring about spare parts.

Dewert User Manual DX1 Series–Updated 05.2021/1 MDR Page 6 of 16
2. Operating manual
2.1 Table design
When designing the table frames, special emphasis was placed on functional and operational safety. The
number of possible pinching points has thus been minimised, while remaining ones have been covered or
protected with spacers to prevent injury, thus ensuring safe and yet simple operation. Nevertheless,
necessary caution must always be exercised when using the table.
The table consists of the following assemblies: - underframe, - scissor section, - upper frame, - upholstery.
Depending on the respective version, these assemblies can feature further attachment parts. The surfaces of
the welded design are plastic coated.
The height adjustment unit is located between the scissor section and the underframe, which guarantees very
high power transmission even in the lowest adjustment range (min. height). By extending or retracting the
lifting tube, the scissors are pushed apart or together, thus enabling adjustment of the lying surface.
The electrical adjustment system does not pose a hazard to the health and safety of the user or the patient
when used as intended. The lifting motor is activated by a low control voltage.
2.2 Height adjustment
Height adjustment (all models with electromotive height adjustment)
To adjust the height, the enclosed foot switch (optionally also hand switch) is operated according to the
marking.
Beforehand, brief activation (double tap) must take place via the foot switch (or the hand switch). Please refer
to section 7. The table is lifted or lowered.
Height adjustment using foot switch rails (optional)
The electric motor for height adjustment is operated by a switch rail attached to the long side of the table,
which can be operated with your foot.
Press the switch rail down = the table is lifted.
Lift the switch rail up = the table is lowered.
Here, too, brief activation (double tap) must take place beforehand (see section 7).
Alternatively, the switch rails can also be led out to the short side of the table. This facilitates
height adjustment from the short sides of the table. The lifting motor is equipped with a freewheeling clutch as
standard. This ensures automatic declutching if an obstacle is encountered when lowering. In other words,
the active tractive force of the motor no longer acts; instead, simply the weight of the upper part of the table is
applied. In the event of unforeseen entrapment, the risk of injury is significantly reduced.
Note on operation
The electric motor is to be operated in intermittent duty mode. This means that a maximum duty cycle
of 25 s must not be exceeded. Before switching the motor back on, an interruption time of at least 400
s must be observed. If the maximum duty cycle is exceeded, an internal thermal switch (protective
temperature limiter) in the motor interrupts the power supply to the actuator. After the electric motor
has cooled down, the thermal switch automatically reconnects the power supply to the actuator.
Height adjustment (with hydraulic height adjustment)
At tables equipped with hydraulic height adjustment, the height is adjusted by repeatedly depressing
(pumping) the foot lever on one side of the table.
To lower the table, the foot lever is simply lifted with your foot.
If the table is only lifted slightly each time the foot lever is depressed after transport or a longer period of non-
use, air bubbles have formed in the hydraulic system. To remove the air bubbles, pump the table upwards
under load and perform an additional 20 - 30 strokes of the pump when the table is in the uppermost position.
This will force air out of the system.
2.3 Upholstery adjustment
Despite the very robust and strong design of the gas spring, the head part must not be used for
sitting!

Dewert User Manual DX1 Series–Updated 05.2021/1 MDR Page 7 of 16
Adjustment of the head part (and foot part for models 1050E, 1050/H and 1095/H)
The head part is adjusted using a gas spring. To operate, press the release lever, which is located at the end
of the head part underneath the upholstery part, in the direction of the upholstered surface. The head part is
lifted slowly to the positive end position. To lower, press down the head part and operate the release lever at
the same time. Once the desired position has been reached, let go of the release lever.
3-sectioned head part (optional)
The 3-sectioned head part consists of an adjustable head part as described under 2.3 and additional
armrests, which can be lowered and removed steplessly, to the right and left of the head part. The clamping is
released by loosening the knurled screw located underneath each armrest. The armrest can now be pulled
down through a range of approx. 180 mm. Re-clamping takes place by subsequently tightening the knurled
screw. To remove the armrests, loosen the knurled screw located directly underneath the head part. The
complete armrest can then be pulled off the head part from the side. The armrests must not be used for
sitting. They only serve to support the patient’s arms.
2.4 Movability (model dependent or optional):
Individually lockable castors
The castors can be locked using the foot-operated brake on the castor housings. In this case, the castors can
neither be moved nor rotated. To release, operate the brake on each castor again.
Please note:
Standard castors are not electrically conductive = optional equipment.
You can recognise conductive castors via the respective marking = yellow dot on the side of the running
surface or a yellow ring.
With this movability option, access to the patient is improved during examination and treatment thanks to easy
positioning within the room. Transporting patients is not considered as intended use.
Central movability
By operating a lever (on the outside of the table feet), all four castors are activated simultaneously. The
following moving options exist:
Stage 1: The castors of the table are locked and can neither be moved nor rotated.
Stage 2: = centre position: The castors are released and can be moved and rotated, the table can be moved
in all directions.
Stage 3: Three castors are released (= can be moved and rotated). The fourth castor is locked and cannot be
rotated (directionally locked castor), i.e. the wheel rolls in a fixed position and helps to steer the table in the
intended direction.
Please note: Rotational movement is only prevented when the castor is swivelled parallel to the lying surface.
This then allows the table to be moved in a straight line without pulling off to the side.
Wheel-lifting/fixing mechanism
The wheel-lifting/fixing mechanism allows a combination between fixed and movable table. There are two twin
castors on each short side of the underframe and foot levers at each corner. The foot levers consist of two
ergonomically arranged counter holders.
This way, the table can be lifted and lowered quietly with your foot. The wheel-lifting/fixing mechanism is not
controlled centrally; a foot lever must be operated on each short side of the table in order to lower the table
onto its feet or to place it on the castors. This option ensures the device can be simply relocated; however,
the device is not intended for transporting patients (ground clearance (distance foot - ground) when moving
the table is approx. 14 mm).
2.5 Additional equipment
Nose opening
If a nose opening is upholstered in the head part as an optional equipment feature, the opening can be closed
with a cushion (optional). To open up the nose opening, reach under the head part when the table is standing
still and press the cushion out from the bottom upwards (a slight amount of pressure is required). To close it,
simply insert the cushion into the opening (a slight amount of pressure is required).
Paper roll holder (optional equipment)
The paper roll holder consists of a holding bar and angle mount brackets or retaining brackets. In addition to the
stainless steel bar, the paper roll holding bar consists of a spring-loaded stainless steel sleeve featuring a round

Dewert User Manual DX1 Series–Updated 05.2021/1 MDR Page 8 of 16
steel gripping disc at the end. To insert the holding bar, feed the guide cotter pin of the stainless steel bar into the
rear hole of the angle mount bracket/retaining bracket. Then push the sleeve with the gripping disc against the
spring force and feed the front guide cotter pin into the second hole. Then relieve the spring tension on the sleeve.
To release the paper roll holder, proceed in the same way.
Side guard (optional):
Laterally lowerable side guard (standard equipment for models 1090/H, 1093/H and 1095/H)
Operating the side guard:
Hold the centre of the rail of the side guard with one hand and release it by moving it minimally sideways
(either towards the head or foot part). At the same time, use your other hand to pull out and turn the locking
pin (red knob) located in the centre of the side guard underneath the upholstery frame. Press down or pull up
the side guard using the rail until the locking pin engages audibly. After the locking pin has engaged, the side
guard is secured in place. To check whether the side guard has engaged properly, move it sideways on the
rail (either towards the head or foot part). Only limited movement should then be noticeable.
Always operate the side guard with the necessary caution. Never operate the side guard if the hands,
fingers, etc. of another person are located between the bars or on the mechanism of the side guard.
Risk of being crushed/pinched!!!
The laterally lowerable side guard is completely screwed in place. If the side guard becomes too loose or has
excessive lateral play over time due to use, it can be readjusted by tightening the screws. The moving parts of
the side guard should be re-lubricated slightly at regular intervals (spray oil, e.g. Ballistol).
Side guard, lowerable, for side rail
This side guard can be placed and fixed on any side rail using a clamp. By loosening the toggle screw of the
clamp, the position and height of the inserted side guard can be shifted and adjusted respectively.
Complete lowering below the upholstery level can only be achieved if the guide points in the direction of the
upholstery when the side guard is inserted into the clamp.
Please note: Never use the side guard as a push handle. It has not been designed for this purpose.
3. Additional accessories (for user-specific table configuration, model dependent)
•Upholstery width 800 mm
•Twin castors ∅ 100 mm, centrally lockable, non-conductive
•Twin castors ∅ 100 mm, individually lockable
•Wheel-lifting/fixing mechanism
•3-sectioned head part
•Side guard
•Paper roll holder
•Additional foot switch or hand switch
•Foot switch fixation on the underframe
•Foot switch rails, for operating the height, laterally or surround
Dewert User Manual DX1 Series–Updated 05.2021/1 MDR Page 9 of 16
4. Technical data
4.1 DX1 series
Model
1000E
1000/H
1030E
1030/H
Max. length (mm)
1950
1950
1950
1950
Width (mm)
700
700
700
700
Head part length (mm)
550
550
750
750
Middle part length (mm)
/
/
/
/
Foot part length (mm)
1400
1400
1200
1200
Weight (approx., depending on
equipment) kg
80
80
85
85
Min. –max. height (mm)
480 to 920 *
470 to 920 *
520 to 960 *
510 to 960 *
Adjustment time (motor) (s)
22
/
22
/
Head part adjustment range
+40° / -35°
+40° / -35°
0° / +75°
0° / +75°
Foot part adjustment range
/
/
/
/
3-sectioned head part adjustment
range
+45° / -25°
+45° / -25°
/
/
Max. patient weight (kg)
225
225
225
225
Model
1050E
1050/H
Max. length (mm)
1950
1950
Width (mm)
700
700
Head part length (mm)
550
550
Middle part length (mm)
480
480
Foot part length (mm)
920
920
Weight (approx., depending on equipment) kg
90
90
Min. –max. height (mm)
500 to 940 *
490 to 940 *
Adjustment time (motor) (s)
22
/
Head part adjustment range
+40° / -30°
+40° / -30°
Foot part adjustment range
0° / + 50°
0° / + 50°
3-sectioned head part adjustment range
45° / -25°
+45° / -25°
Max. patient weight (kg)
225
225
Model
1090/H
1093/H
1095/H
Length (mm)
1950
1950
1950
Width (mm)
700
700
700
Head part length (mm)
550
750
550
Middle part length (mm)
/
/
480
Foot part length (mm)
1400
1200
920
Overall length (mm)
2060
2060
2060
Overall width (mm)
780
780
780
Weight (approx., depending on equipment) kg
100
105
110
Min. –max. height (mm)
500 to 950
540 to 990
520 to 970
Head part adjustment range
0° / + 65°
0° / +75°
0° / + 65°
Foot part adjustment range
/
/
0° / + 50°
Max. patient weight (kg)
225
225
225
Technical data subject to change
*for movability art. no.046: Height plus 20 mm
for movability art. no. 040N: Height minus 10 mm

Dewert User Manual DX1 Series–Updated 05.2021/1 MDR Page 10 of 16
The motor is equipped with a thermal protective switch as standard. This causes the motor to cut out if the weight
load is too extreme or the motor’s duty cycle (DC: 25 s/400 s) is exceeded. The table should be ready for use again
after a 15-minute rest period. There is, therefore, no risk of overloading the motor.
The mechanics of the tables have been designed with extensive safety margins in mind.
The max. patient weight is provided with a 4-fold static safety factor, i.e. the design has been fully tested for a 4-
fold load.
4.2 Technical data for electric motor
Manufacturer: Hanning Elektro-Werke GmbH & Co, D-33813 Oerlinghausen
Type of motor: SL 95
Type of actuator: Brushless asynchronous industrial motor
Mode of operation: Electromechanical linear motor with maintenance-free lifetime lubrication
Intermittent duty –installed thermal switch
Electronic activation with internal supply for the control element
Duty cycle (DC): 25 s / 400 s
i.e. move the table for max. 25 seconds under nominal load, then observe
a break of at least 400 seconds.
Nominal voltage: 220 –240 V, 1-50/60 Hz
Nominal consumption: 850 W
Current consumption: 3.7 A
Protection class: II (protective insulation), connecting cable without protective conductor
Protection rating: IPX4 –resistant to water splashes,
Degree of protection: B
The motor is maintenance free.
When operated with sinusoidal alternating voltage, the motors used do not cause field or line-borne interference
within the meaning of EN 50081, Part 1 and 2, nor can their function be impaired by electromagnetic influences
within the meaning of EN 50082, Part 1 and 2.
4.3 Technical data for hydraulic system
Manufacturer: Power-Packer Europa B.V., NL-7575 AT Oldenzaal
Type: Compact MK5 long
Mode of operation: Hydraulic cylinder with pump
The hydraulic unit is maintenance free.
5. Cleaning instructions
Upholstery covering
There are two different collections of upholstery covering to choose from:
•Skai Pandoria Plus (manufactured by Hornschuch/Continental in Germany)
•Skai Toronto EN (manufactured by Hornschuch/Continental in Germany)
•
For cleaning and disinfection, a selection of agents from various manufacturers has been tested for
compatibility. Please refer to the separate enclosed sheet.
The table is equipped as standard with the Dewert hygiene standards, which enable optimal cleaning and
disinfection:
•Hinge lids are made of the identical upholstery covering
•All undersides of the upholstery parts are coated with an upholstery covering and can thus be cleaned
and disinfected

Dewert User Manual DX1 Series–Updated 05.2021/1 MDR Page 11 of 16
•Vent holes underneath the upholstery:
For the homogeneous foam to recover quickly, a rapid exchange of air is necessary. To ensure this, there
are individual vent points on the underside of the upholstery, which are hygienically sealed with special air
compensation caps that assume a valve function.
•Easy hygiene due to an open design
•Optional: Upholstery covering Skai Toronto EN with staynu
Table frame
The plastic-coated table frame can be cleaned with mild household cleaners, if necessary. Do not use
aggressive, abrasive or corrosive agents. After cleaning, dry the frame with a soft dry cloth.
Seal deep scratches and worn areas with suitable repair agents to prevent moisture penetration.
Important:
At tables with hydraulic height adjustment as well as for the gas springs, wipe the piston rod with a soft cloth
at regular intervals. This prevents dust from entering through the dust lip and preserves the service life of the
unit.
Please note:
When cleaning, secure the table against unintentional lowering of the lying surface.
To do so, set all the adjustable sections to the horizontal position.
At tables with electromotive height adjustment, disconnect the power plug from the mains socket
beforehand.
At tables with hydraulic height adjustment, block the foot levers.
The power plug must not come into contact with water or cleaning agents.
The electrical components must not display any external damage through which liquid could enter. Do not
clean the table with water jets, a high-pressure cleaner or with a so-called bed washer. Only use moist cloths.
6. Maintenance and technical inspection
The device has been designed and manufactured in such a way that it will operate safely over a very long
period of time if used as intended and in a correct manner. Depending on the conditions of use, place of use
and care, the expected operating service life is 10 years or 100,000 drive cycles (double stroke = 1x up and
down).
Regular maintenance procedures are required to ensure patient, user and product safety. They must be
carried out every two years at the latest.
The maintenance procedures can be carried out by qualified personnel/instructed personnel. The scope of
maintenance includes, for example:
•Thorough visual inspection of all components, especially motor and switch with mains supply line or
hydraulic system
•Function check
•Check all swivel joints for completeness.
•Check proper fit of the screw connections.
•Lubricate the swivel joints and operating levers slightly with low viscosity spray oil, if necessary.
•Lubricate the roller guides with a little bearing grease or similar using a brush, if necessary.
Insufficient lubrication is noticeable through noise development.
A checklist for maintenance/technical inspection can be found in the appendix.

Dewert User Manual DX1 Series–Updated 05.2021/1 MDR Page 12 of 16
In addition to maintenance in accordance with the legal requirements of DGUV Regulation 3 / IEC
62353, a technical inspection must be carried out every two years at the latest on tables with
electromotive adjustment.
This technical inspection may only be carried out by authorised and trained specialists.
A checklist for maintenance/technical inspection can be found in the appendix.
Despite regular maintenance/technical inspection, the user is also responsible for patient safety and proper
and reliable functioning.
As the user, make sure that the table is in good working order prior to each use (visual inspection). In the
event of any abnormalities, take the table out of service immediately and inform the operator.
Replace damaged or worn components immediately and do not use the table until it has been repaired.
The table complies with the safety regulations prescribed at the time it was placed on the market. Improper
repairs and structural modifications (disassembly of original parts, installation of third-party parts, etc.) may
result in hazards for patients and users. In the event of uncoordinated modifications to the table, the
Declaration of Conformity loses its validity and the warranty shall become null and void.
We cannot be held liable for damages resulting from uncoordinated modifications.
Only original Dewert spare parts may be used.
Risk of electrocution!
Work on the electrical system may only be carried out by qualified and authorised personnel in
compliance with all relevant provisions and safety regulations!
Foot and hand switches for adjusting the electric motor as well as gas springs are wearing parts whose
function may be impaired over the years depending on the frequency of use.
Both can be replaced without too much effort. Please request the corresponding installation diagram, if
required.
Replacement parts can be obtained directly from Dewert.
7. Safety devices
Tables with electromotive adjustment must have an automatic device to deactivate the control elements when
moving the table. Reactivation of the control elements must be designed in such a way that they cannot be
triggered accidentally by patients, users or third parties.
The actuator of this table is equipped with an integrated safety device to protect against
unauthorised/unintentional operation. This device goes into “sleep mode” 3 seconds after the last operation
and can only be reactivated with a defined switching sequence, the so-called double tap. To “wake up” the
actuator or the control unit, first press the desired direction of travel for approx. 1 second on the control
element. After a short waiting time (1-2 seconds), press the desired direction of travel again and the actuator
can be moved for a maximum of 30 seconds in this direction of travel. If the switching cycle of the double-tap
function is not observed, the actuator cannot be operated. After 30 seconds of operation in one direction of
travel, the actuator switches off and goes into sleep mode. The actuator can still be operated for up to 3
seconds after the last operation to ensure fine adjustment. Within this time window, it is possible to move in
each direction of travel again for a maximum of 30 seconds. The actuator always goes into “sleep mode”
automatically 3 seconds after the last operation.
The motor is equipped with a safety freewheeling clutch as standard:
When lowering, the motor declutches automatically if an obstacle is encountered, and automatic declutching
is interrupted. In other words, the active tractive force of the motor no longer acts; instead, simply the weight
of the upper part of the table is applied. In the event of unforeseen entrapment, the risk of injury is significantly
limited.

Dewert User Manual DX1 Series–Updated 05.2021/1 MDR Page 13 of 16
8. Reporting obligation
All serious incidents occurring in connection with the product shall be reported to the manufacturer (K.H.
Dewert GmbH) and to the competent authority of the Member State in which the user and/or the patient
resides.
Member State
Competent authority
Web
Estonia
Health Board
Terviseamet
https://www.terviseamet.ee/en
Finland
Fimea
https://www.fimea.fi/web/en
Iceland
Iceland Medicines
Agency
https://www.ima.is/
Luxembourg
CNS
https://cns.public.lu/en
Malta
MCCAA
https://mccaa.org.mt/
Switzerland
Swiss medic
https://www.swissmedic.ch/swissmedic/de/home.html
A serious incident is an incident that directly or indirectly had, could have had, or may have had any of the
following consequences:
•death of a patient, user or other person,
•temporary or permanent serious deterioration of the state of health of a patient, user or other person,
•serious threat to public health.
9. Disposal
•Packaging
Safety notice: Pay attention to sharp edges and pointed objects during disposal!
The packaging materials produced are mainly:
oCardboard/Paper
oPlastic
oWood (when delivered on a pallet)
Please observe the local regulations for waste disposal and preferably recycle the materials.
As a manufacturer, we are licensed as a participant in the Dual System in accordance with the German
Packaging Act (VerpackG) and therefore bear the disposal costs, meaning you can dispose of the packaging
free of charge.
•Product
Safety notices:
oPay attention to sharp edges and pointed objects!
oWhen transporting the table, only take hold of the underframe, NOT the upper frame.
oIn order to prevent accidents later on, the no longer used product must be rendered unusable
immediately, e.g. by disconnecting the power cable.
Please observe the local regulations for waste disposal and preferably recycle the materials.
Tables with electromotive height adjustment are subject to WEEE Directive 2012/19/EU. These old devices
must be collected, recycled and disposed of in an environmentally sound manner. Use the return and
collection systems available to you for this purpose.

Dewert User Manual DX1 Series–Updated 05.2021/1 MDR Page 14 of 16
10. Declaration of Conformity
EU Declaration of Conformity for medical devices
Manufacturer: K.H. DEWERT GmbH
Vollmestr. 7
D-33649 Bielefeld
The product: Height adjustable table
Model designation: DX1 series –models:
1000E; 1000/H
1030E; 1030/H
1050E; 1050/H
1090/H; 1093/H; 1095/H
The numerical codes -00, -03, -04 appended to the individual model designation only indicate the colour of the frame
(-00 = white frame, -03= white aluminium frame, -04= grey aluminium frame).
Basic UDI-DI: 4063907KHDewertLiegenP2
SRN: DE-MF-000005967
Intended purpose:
The table is used for the ideal positioning of patients for the purpose of curative and disease treatment,
examination, massage and health therapy.
Table operation and patient positioning on the table may only be performed by professionally trained persons
who have been instructed in its use or who, through experience with other similar medical devices, have
knowledge of its proper use, taking into account possible hazards.
Equipped with the options of movability (not wheel-lifting/fixing mechanism), side guards and push handle, the
intended purpose of the table is extended and also provides for the ideal positioning of patients for the
purpose of transport to pre-treatment or post-treatment locations. Patient positioning during the recovery
phase after a medical procedure is also permitted under supervision.
Otherwise, the device must only be moved within the room for cleaning or patient access.
This device has been developed exclusively for use indoors and in normal ambient conditions and can be
used in the following areas: laboratories, medical practices, examination and treatment rooms, hospitals,
clinics, physiotherapy practices, occupational therapy centres and doctors’ surgeries.
This table is not classed as surgical furniture and must, therefore, not be used for surgical purposes.
complies with the relevant provisions of Regulation (EU) 2017/745, Article 19, Annex IV (Class 1
according to Annex VIII, Chapter III, No. 4.1 of 5 April 2017).
We hereby declare conformity with the aforesaid directive.
As the manufacturer, we bear sole responsibility for issuing this EU Declaration of Conformity.
Mark:
Bielefeld/Germany, 26 May 2021
K.-H. DEWERT GmbH
Management
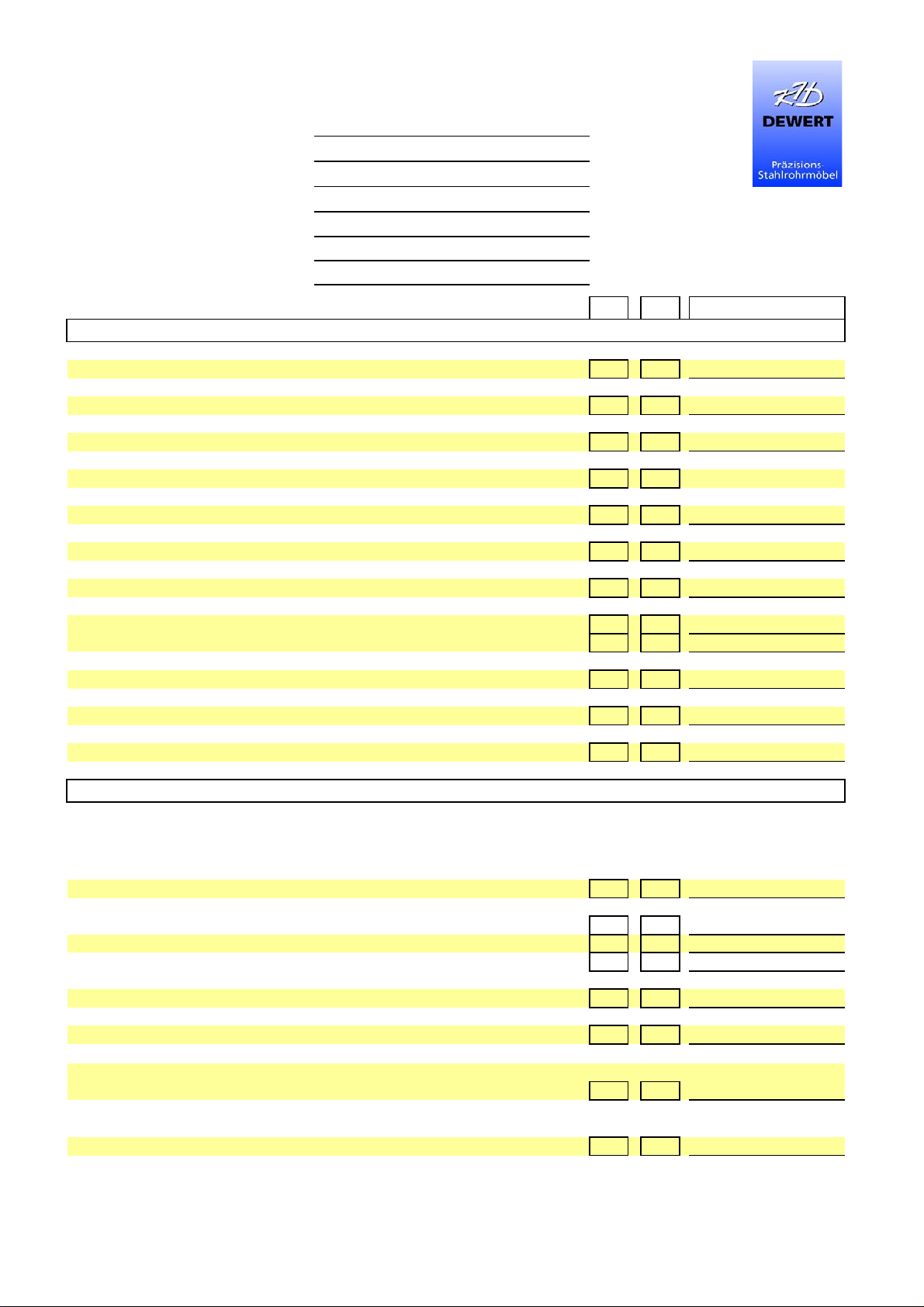
Checklist for maintenance/technical inspection
to IEC 62353 and DGUV Regulation 3
Device
Model designation
Serial no.
Location
Responsible person
Date, inspector
Actuator designation
Inspections OK FAIL Description of defects
V
isual inspection
Overall impression of the table OK?
Labels, CE mark, type plate present?
Manufacturer’s operating manual available and accessible?
Sufficient space available when carrying out all adjustment functions?
Mechanical structure undamaged:
Welds without obvious damage?
Screw connections correct and complete?
Upholstery undamaged?
Upholstery attachment correct?
All mechanical elements intact and complete?
Electrical system and power cable undamaged?
All switches and supply lines undamaged?
Function check
With electromotive adjustment:
Move all motors with the foot switch or the hand switch to both limit positions until
automatic switch-off to ensure that:
* the table operates smoothly without any collisions or blockages
* no cable/connection can be overstretched, crushed or otherwise
damaged
* the motors run without noticeable noise development
* the end position switch-off of the actuators works properly
Additionally:
Power cables and power plugs undamaged?
Correct and safe routing of the power cable and connection?
Checking the safety device:
Double-tap function and freewheeling motor, function given?
With hydraulic height adjustment:
Function given?
Check by operating the pedals until the table reaches the uppermost position
Continue to operate the pedals approx. 5-10 times (any air is pressed out of the system)

OK FAIL Description of defects
Height retained?
Hydraulic pump leaking?
Smooth lowering possible?
Check by operating the pedals to lower the table
Strong noise development?
Wipe the piston rod with a cloth
Lying surface adjustment functions:
Metal ratchets - arrester - gas spring
Metal ratchet inspection: Lifting the lying surface segment:
Do the two metal ratchets engage properly and securely?
Do they engage evenly?
Is this ensured in every adjustment position?
Arrester inspection: Lifting the lying surface segment:
Is the segment held properly and securely at every height?
Perform the test also with a load
Is the function smooth without clamping?
(= move the lying surface segment without fixing the clamping lever)
Gas spring inspection: Lifting the lying surface segment:
Does the gas spring respond when released?
Is the segment held properly and securely at every height?
Is the piston rod of the gas spring free of grease and not leaking?
Clean the piston rod with a cloth
Accessories:
Accessories such as belts, belt pads, belt guides, paper roll holders,
armrests, etc. undamaged, and is proper and secure fixing/function possible?
All necessary toggle screws present?
Possible movability:
Castors undamaged, freewheeling given?
Connection to frame undamaged?
Re-tighten all screw connections (with central locking system,
also the grub screws of switch levers)
Safe braking effect?
Check with locked brakes by pulling and pushing the table
Structural inspection:
Checking the scissor fittings:
Use a wrench to check the six fixing screws of the scissors (remove the black caps for this purpose)
and the hexagon socket head screws for a very tight fit
Checking the side guard:
Easy adjustment possible without clamping?
Proper locking during setup/lowering?
Laterally lowerable side guard:
No adjustment possible without pulling the locking knob?
Proper engagement in the end positions?
Electrical testing
Leakage current measurement (protection class II, degree of protection B)
(max. 0.1 mA permissible) Measured value:
Final assessment
Everything error free?
Device put out of operation until it has been repaired?
Comments
Place / Date / Signature of inspector
Next inspection
This manual suits for next models
9
Table of contents
Other Dewert Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual