DIATECH PHARMACOGENETICS EasyPGX qPCR instrument 96 User manual

www.diatechpharmacogenetics.com
ACCESSORY
EasyPGX®qPCR instrument 96
User manual –version 2017/06
For in vitro diagnostic use
Exclusively if used in combination with specific in vitro diagnostic kit (IVD) commercialized by
Diatech Pharmacogenetics srl requiring the use of “EasyPGX®qPCR instrument 96”in the
respective user manual
RT800-96
8057289090675
2017/06
Diatech Pharmacogenetics srl a Socio Unico
Società soggetta all’attività di direzione e coordinamento di Diatech srl con sede in Jesi
Via Ignazio Silone, 1 b - 60035 Jesi (AN) Italy
C.F./P.Iva/R.Imprese di Ancona n. 02483840423
Tel. +39 0731 213243 Fax +39 0731 213239
info@diatechpharmacogenetics.com www.diatechpharmacogenetics.com

EasyPGX® qPCR instrument 96
Cat.Nr: RT800-96
2/21
2017/06
This page was intentionally left blank

EasyPGX® qPCR instrument 96
Cat.Nr: RT800-96
3/21
2017/06
Index page
INTENDED USE 5
CONTENT 5
MATERIALS REQUIRED BUT NOT PROVIDED 5
DOCUMENTS AVAILABLE ON-LINE 5
SYMBOLS 6
PRODUCT USE LIMITATIONS 6
QUALITY ASSURANCE 6
WARNINGS AND PRECAUTIONS 6
SAFETY INFORMATION 7
ELECTRIC SAFETY 7
FLUIDS AND REAGENT 7
DANGER OF BURNS 7
OPERATIONAL ENVIRONMENT REQUIREMENTS 7
ELECTROSTATIC DISCHARGES 7
OTHER FEATURES 7
INSTALLATION 8
POSITION OF THE INSTRUMENT 8
UNPACKING OF THE INSTRUMENT 8
CLEANING THE HEATING BLOCK 8
CONNECT THE INSTRUMENT TO THE ELECTRIC POWER 8
CONNECT KEYBOARD AND MOUSE TO THE INSTRUMENT (OPTIONAL) 9
BACKGROUD CALIBRATION 9
DATE AND TIME SETTING 9
DIAGNOSTIC TEST 9
SOFTWARE INSTALLATION 10
“ARIA DX” SOFTWARE INSTALLATION 10
HARDWARE FUNCTIONS 13
LED INDICATOR 13
LOADING THE HEATING BLOCK 13
SOFTWARE FUNCTIONS 13
IMPORT OF TEMPLATES 13
EXECUTION OF A RUN 13

EasyPGX® qPCR instrument 96
Cat.Nr: RT800-96
4/21
2017/06
EXPORTING DATA 14
ANALYSIS OF THE RESULTS 14
CALIBRATIONS 15
MOTOR CALIBRATION 15
BACKGROUD CALIBRATION 15
TOUCH SCREEN CALIBRATION 15
INSTRUMENT MANTEINANCE 15
CLEANING THE INSTRUMENT 15
CLEANING THE OPTICAL MODULES 15
TECHNICAL SPECIFICATIONS 17
WARRANTY AND CLAUSE OF LIABILITY 18
WARRANTY 18
EXCLUSIONS 18
GUARANTEE LIMITATION AND REMEDIES 18
RESPONSIBILITY CLAUSE 18
APPENDIX A: ELECTRIC AND ELECTRONIC WASTE DISPOSAL DIRECTIVE (WEEE) 19
OPERATOR NOTES 20

EasyPGX® qPCR instrument 96
Cat.Nr: RT800-96
5/21
2017/06
INTENDED USE
“EasyPGX®qPCR instrument 96”is a tool for quantitative amplification, detection and analysis of nucleic acids. The
system is designed to combine thermocycler, optical system with LED excitation source and data analysis software.
“EasyPGX®qPCR instrument 96”is validated in combination with "EasyPGX®ready" kit, manufactured and
commercialized by Diatech Pharmacogenetics srl. For further details, refer to the user manuals available in the reserved
area of the web site www.diatechpharmacogenetics.com/area-riservata.
CONTENT
“EasyPGX®qPCR instrument 96”consists of:
EasyPGX®qPCR instrument 96
(complete with power cord)
1 pc
RT800-96_Accessory set
1 pc
Strip holder
1 pc
MATERIALS REQUIRED BUT NOT PROVIDED
USB pen drive
USB 2.0 or higher (if used also for execution of runs)
Personal computer
Minimum requirements for running Aria Dx software:
▪Operating system: Windows 7 (Professional and Ultimate editions) or Windows 10
▪Supported architectures:
ox86 (32 bit) supported on Windows 7
ox64 (64 bit) supported on Windows 7 and 10
▪Programs:
oMicrosoft .NET Framework 4.0
oMicrosoft SQL Server 2012 (only required for ET software) Runtime Components of
Microsoft Visual C ++ 2010 Libraries
▪Processor: 2 GHz Dual Core Processor
▪Working memory (RAM): 2 GB (better if higher)
▪Hard drive space: 40 GB
▪Display resolution: 1024 × 768 (1280 × 1024 recommended)
DOCUMENTS AVAILABLE ON-LINE
The following documents are available in the reserved area of the web site www.diatechpharmacogenetics.com/area-
riservata:
▪User manual “EasyPGX®qPCR instrument 96”–instructions for use
213243).
EasyPGX® qPCR instrument 96
Cat.Nr: RT800-96
6/21
2017/06
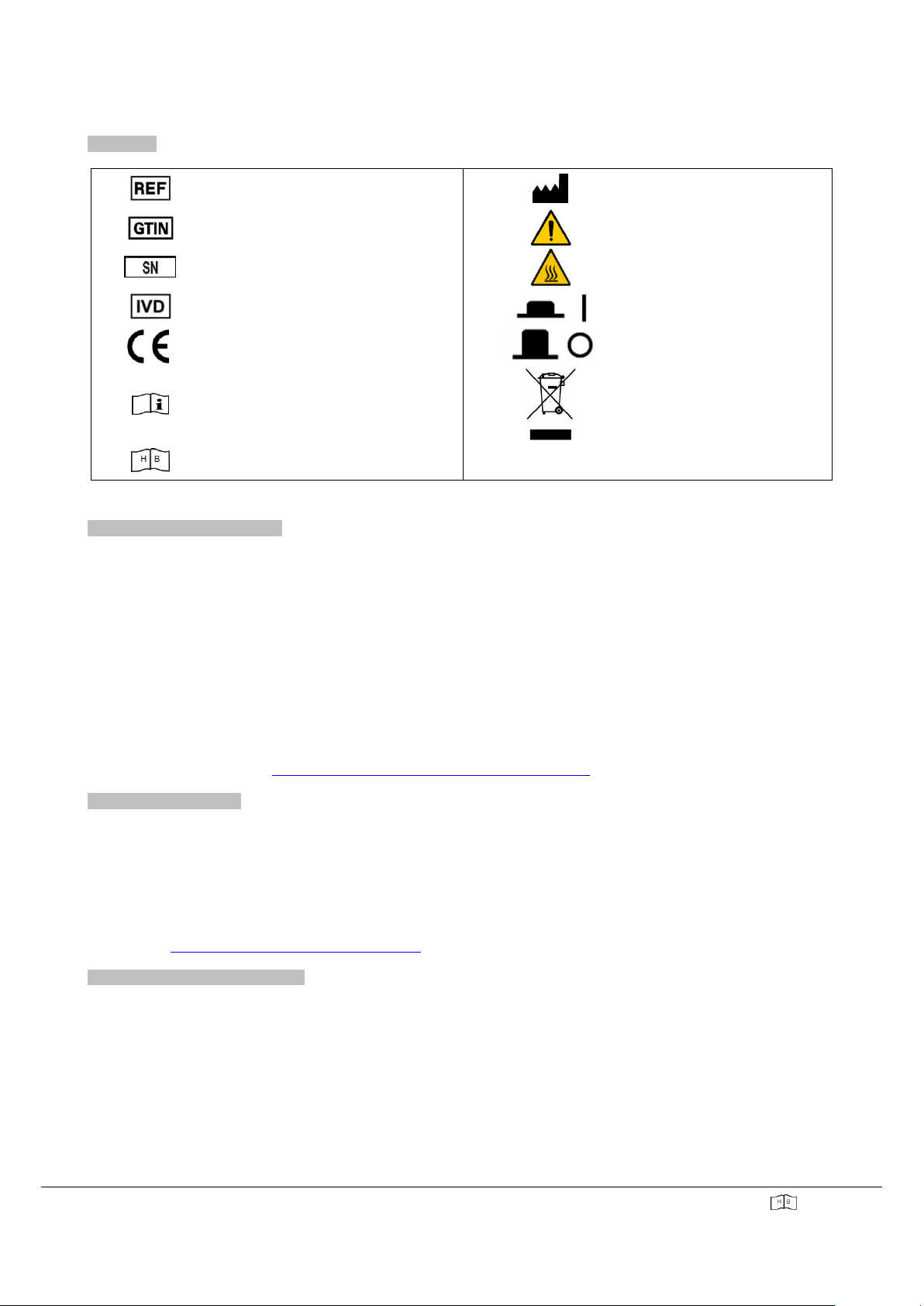
SYMBOLS
Catalogue number (product code)
Manufacturer
Global Trade Item Number
Attention!
Serial number
Hot surface
For in vitro diagnostic use
Instrument switched on
European Community
Instrument switched off
Consult the user manual
WEEE mark for Europe
User manual (handbook)
PRODUCT USE LIMITATIONS
▪“EasyPGX®qPCR instrument 96”can only be used by specialized personnel, properly instructed and trained.
▪It is necessary to operate in compliance with the general guidelines of Good Laboratory Practice (GLP) and the
instructions contained in this manual.
▪“EasyPGX®qPCR instrument 96”has been validated for use in combination with the "EasyPGX®ready" kit,
manufactured and commercialized by Diatech Pharmacogenetics srl. The reliability of the results also depends on
the procedures performed in the preliminary phases of the amplification, including the selection of the starting
biological samples, the storage of the samples and the extraction of the nucleic acids.
▪The results obtained using “EasyPGX®qPCR instrument 96”, in combination with "EasyPGX®ready" kit, are not a
diagnostic report, they must be interpreted by a professional expert, with reference to the clinical history of the
patient, his familiar history and other laboratory tests, in the context of a comprehensive clinical evaluation. Diatech
Pharmacogenetics assumes no responsibility for the clinical decisions taken.
▪“EasyPGX®qPCR instrument 96”is covered by CE marking, according to the European Directive 98/79/EC on in
vitro diagnostic medical devices (IVD), only in countries that accept the user manual translated into the languages
available on the web site www.diatechpharmacogenetics.com/area-riservata.
QUALITY ASSURANCE
▪The “EasyPGX®centrifuge/vortex 1.5 ml”kit has been designed, developed and validated in compliance with the
Directive 98/79/EC on in vitro diagnostic (IVD) medical devices, transposed in Italy in the D.Lgs No 332/2000 and
subsequent legislative changes, and in accordance with the procedures of the Company’s Quality System certified
for conformance to the European regulatory standards EN ISO 9001 and ISO 13485.
▪The consistent product quality of “EasyPGX®qPCR instrument 96”is guaranteed by the application of a tight
quality control process on operating procedures for the realization of the product and subsequent management to
the customer. The quality of each lot is attested in the Certificate of Analysis available upon request to the Customer
WARNINGS AND PRECAUTIONS
1. Carefully read this User Manual.
2. If you are not going to use the instrument for an extended period of time, disconnect the power cord from the wall
outlet.
3. The instrument can operate at temperatures between +20°/+30 °C (+65 /+95°F). Do not subject the instrument to
repeated temperature changes.
4. Do not subject the instrument to relative humidity greater than 80% for a prolonged period of time.
5. Any intervention on the instrument can only be performed by Diatech Pharmacogenetics personnel.
Diatech Pharmacogenetics is exempted from any liability if repairs or modifications are made by unauthorized
personnel.
6. Carry out the procedures for using the instrument and its maintenance according to the Good Laboratory Practice
(GLP) general guidelines.

EasyPGX® qPCR instrument 96
Cat.Nr: RT800-96
7/21
2017/06
SAFETY INFORMATION
This product is designed for easy and reliable use in compliance with accepted safety standards. Use does not involve
any risks if it is in accordance with the instructions contained in this document. However, misuse may result in damage to
the equipment or cause health hazard. It is important that the following safety precautions are read and understood
before using the instrument.
All users should read and understand this user manual and use the unit only in accordance with the instructions
provided. Failure to follow the instructions may cause the protection provided by the instrument to be compromised.
Pay particular attention to the paragraphs marked with this symbol.
ELECTRIC SAFETY
7. Connect the instrument only to an appropriate voltage circuit (100-240 VAC).
8. Make sure that the power cord and socket are easily accessible while using the instrument so that the instrument
can be easily disconnected if necessary.
9. Do not connect the instrument to a socket without proper electrical grounding.
10. Do not touch any electrical switch or socket with wet hands.
11. Switch off the instrument before disconnecting it from the electrical socket.
12. Disconnect the instrument from the socket before any cleaning or maintenance work.
13. If liquid infiltrates the unit, disconnect the power socket and contact the supplier.
14. Do not connect the instrument to the same socket where other instruments with high electrical consumption (e.g.
refrigerators or freezers) are connected.
15. Maintenance or repair activities that involve electrical components can only be performed by qualified personnel.
FLUIDS AND REAGENT
▪Fill the reaction tubes/strips/plates keeping the safety distance from the instrument to prevent accidental spills of
liquids on the instrument.
▪Do not use the instrument to process inflammable, explosive or reactive substances.
▪It is responsibility of the user to apply appropriate safety measures if potentially pathogenic material, radioactive
substances or other hazardous substances are used.
▪Do not immerse the instrument in any liquid.
DANGER OF BURNS
▪Do not touch the thermoblock, the inner part of the lid or the reaction tubes/strips/plates while the instrument is
operating. These areas can quickly reach temperatures greater than 50°C. Keep the lid closed until the temperature
has fallen below 30°C.
▪Do not use any material (tubes, foil, ...) that is not sufficiently stable at high temperatures (up to 120°C).
OPERATIONAL ENVIRONMENT REQUIREMENTS
▪The fans placed on the hull of the instrument must always be free from obstructions and free to operate. Always
leave at least 10 cm of space around the instrument.
▪Keep the environment at a temperature between 20 and 30 °C and between 20 and 80% relative humidity.
▪Do not use the instrument in environments where aggressive or explosive volatile mixtures are present. Contact
Diatech Pharmacogenetics support if you need to use the instrument in non-conventional environment
ELECTROSTATIC DISCHARGES
Electrostatic discharges greater than 8000 volts can interfere with the functionality of USB ports on the instrument. It is
necessary to handle the instrument carefully if you are in a highly electrostatic environment by applying appropriate
antistatic measures before touching the instrument. ESD STM5.1- 1998 Class 3B.
The “EasyPGX®qPCR instrument 96”complies with IEC 61326- 2-6 standards regarding electromagnetic compatibility
for IVD medical equipment. The instrument has been designed and tested for CISPR 11 Class A. In a domestic
environment it can cause radio interference, in which case it may be necessary to take measures to mitigate the
interference. It is advisable to choose an appropriate electromagnetic environment for the instrument before use.
OTHER FEATURES
▪Degree of pollution 2
▪Installation category II
▪Use only in closed environments

EasyPGX® qPCR instrument 96
Cat.Nr: RT800-96
8/21
2017/06
INSTALLATION
POSITION OF THE INSTRUMENT
Locate a solid, stable, flat, and clean surface for the instrument. Make sure that:
▪The instrument is completely stable.
▪The slots of the fans positioned on the back of the instrument are perfectly free.
▪The instrument is at least 10 cm apart on each side from walls or other instruments.
▪The instrument isn’t close to anything that can cause vibrations.
▪The ambient temperature is between 20 and 30°C with relative humidity between 20 and 80% (no
condensation).
▪The atmosphere is not explosive.
UNPACKING OF THE INSTRUMENT
▪Make sure that the box containing the instrument is upright, as shown below.
▪Cut the plastic straps and open the box.
▪Remove the accessories and lift the cardboard box. The instrument stays supported on the box.
▪Lift the instrument and place it where it is expected.
The instrument weighs 23 kg. To avoid damages to the instrument and people, it is recommended to lift the
instrument at least in two.
CLEANING THE HEATING BLOCK
Whit the instrument cover opened, clean the outer and inner surfaces of the heating block:
▪Lift the heat block door by pulling the handle forward and then upwards until it is removed from the instrument.
▪Clean the wells by using a compressed air bottle. Spray the air on the block from a distance of 8-10 cm.
▪Dampen a cloth that does not leave residues with deionized water and gently wipe the lock and the inside of
the door. Close the door and clean the top.
▪Close the instrument cover.
CONNECT THE INSTRUMENT TO THE ELECTRIC POWER
▪Insert the power cord into the socket located on the back of the instrument.
▪Insert the power cord into the electrical socket.
Refer to the "Security Information" section before connecting the instrument to the power supply.

EasyPGX® qPCR instrument 96
Cat.Nr: RT800-96
9/21
2017/06
CONNECT KEYBOARD AND MOUSE TO THE INSTRUMENT (OPTIONAL)
The instrument has a touchscreen display that allows it to be used without the need to connect any additional accessory.
However, it is possible to connect a mouse and a keyboard to the instrument using the USB ports on both the front and
rear of the instrument.
BACKGROUD CALIBRATION
▪Switch on the instrument by pressing the button on the bottom of the front panel.
▪The instrument automatically performs a series of checks at each power up to ensure basic hardware
functionality. If an error is detected, perform a more accurate diagnostic test (see section "Performing a
diagnostic test"). When the checks are complete, the touchscreen display will show the Home screen.
▪On the Home screen, press "Settings", then "System Settings" and finally "Backgroud Calibration".
▪Select the optical module you want to calibrate and press "Calibrate". A window opens which contains
instructions for loading a 96-well plate containing 20 μl of deionized water or TE buffer in each well of the heat
block.
To run the "Backgroud Calibration", use the dedicated consumables included in the RT800-96 Accessory set.
▪Prepare the plate and load it into the heat block. Press "OK" to launch the test.
▪At the end of the procedure, on the display appears a window that returns the result of the test, click the "OK"
button to close the window.
If the "Backgroud Calibration" procedure fails, contact Diatech Pharmacogenetics Technical Support (email:
[email protected]; phone: +39 0731-213243).
DATE AND TIME SETTING
▪In the lower-right corner of the display, press the displayed date and time.
▪A menu window shows up, press "Change Date and Time".
▪In the window that appears, set the correct date and time. Click on the "Help" icon to see detailed instructions
for setting the date and time.
DIAGNOSTIC TEST
▪On the Home screen, press "Settings", then press "Instrument Diagnostic".
▪Press "Run Diagnostic".
▪Tick the "All Test" box and press "Run". A window opens that prompts you to make sure that no plate is
loaded on the heating block.
▪Press "OK" to continue. A window that displays the installed optical modules opens.
▪Check the boxes for the installed optical modules (FAM, HEX), and leave boxes for the other unopened optical
modules. Press "OK".
▪The instrument begins to perform the scheduled tests. The first test is about interaction with the user.
▪During this test, follow the instructions that appear on the display and answer the questions that are displayed.
At the end of this initial test, the procedure continues automatically without needing to interact with the user.
▪At the end of the "Diagnostic Check", the display shows "Diagnostic Report".
▪Verify at the beginning of the report the number of successful tests performed on the total of tests, as shown in
the image below.
▪Close the report, the instrument installation is complete.
If one or more tests fail, contact Diatech Pharmacogenetics Technical Support (email:
[email protected]; phone: +39 0731-213243).
EasyPGX® qPCR instrument 96
Cat.Nr: RT800-96
10/21
2017/06
SOFTWARE INSTALLATION
The Aria DX software is validated to be used in combination with "EasyPGX®ready" kits manufactured and
commercialized by Diatech Pharmacogenetics srl. Verify that the version you are using corresponds to that
reported in the "EasyPGX®ready" kits user manuals, otherwise contact Diatech Pharmacogenetics srl
The instrument can be connected to a dedicated PC or work stand-alone using a USB stick. If you want to
connect the instrument to a PC, contact Diatech Pharmacogenetics srl support via email
software on the computer that will be used for the analysis of the results.
To check the minimum requirements of the PC, refer to the section "Material Required but Not Provided".
The standard Windows Installer setup (.msi) is available on request from Diatech Pharmacogenetics
Such software can be installed on any computer to run the session analysis.
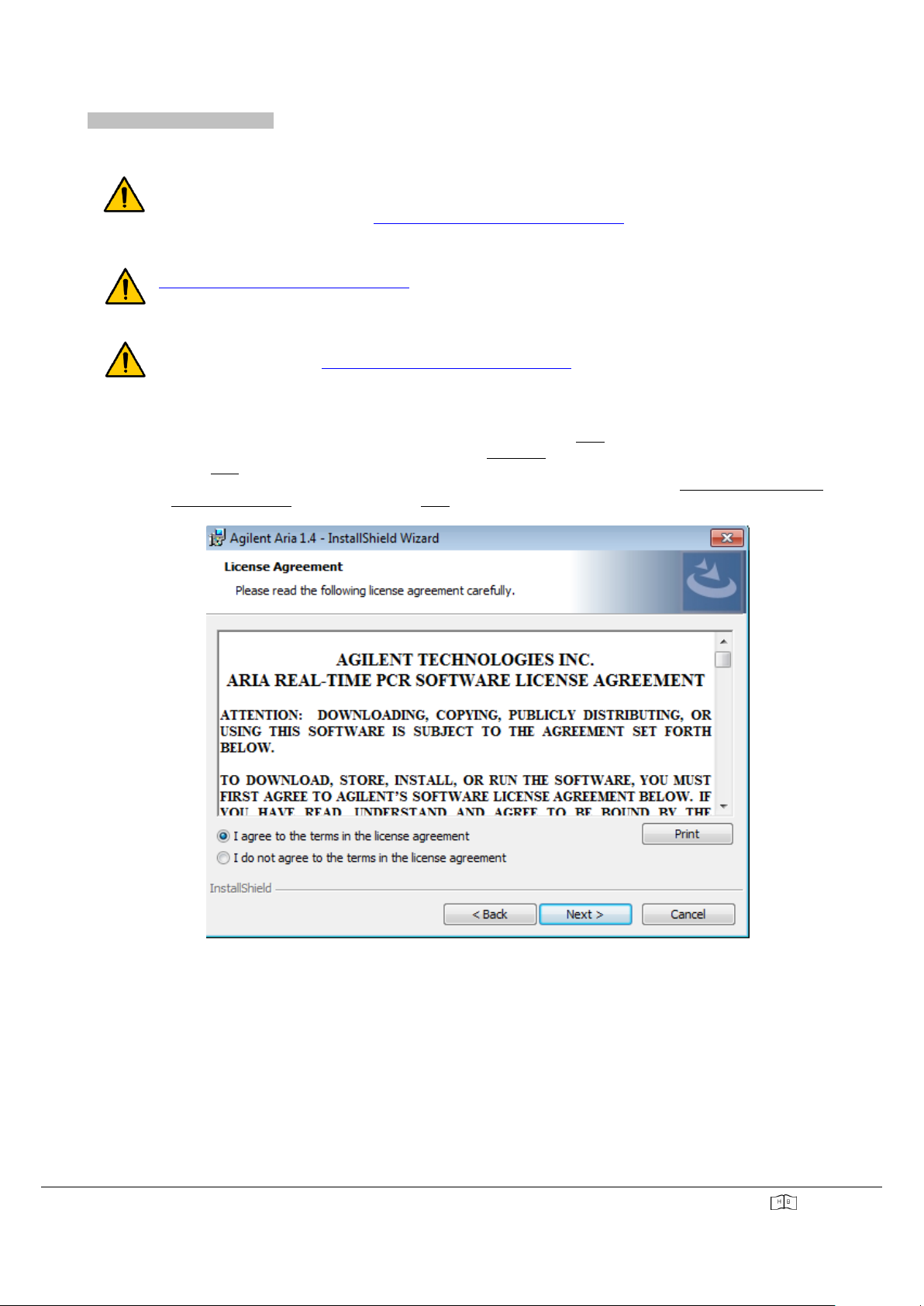
“ARIA DX”SOFTWARE INSTALLATION
▪To launch the installation, double-click on the installer and press "Next".
▪Once the files are extracted, the wizard opens the "Welcome" window.
▪Click "Next" to continue the installation.
▪Read the software license agreement that appears on the screen and then select "I agree the terms in the
License Agreement" to continue. Press "Next".

EasyPGX® qPCR instrument 96
Cat.Nr: RT800-96
11/21
2017/06
▪The "Application Mode" window opens, select "AriaDX" and press "Next".
▪The "Setup Type" window opens. Select "Standard" and press "Next".
Do not change this selection
EasyPGX® qPCR instrument 96
Cat.Nr: RT800-96
12/21
2017/06
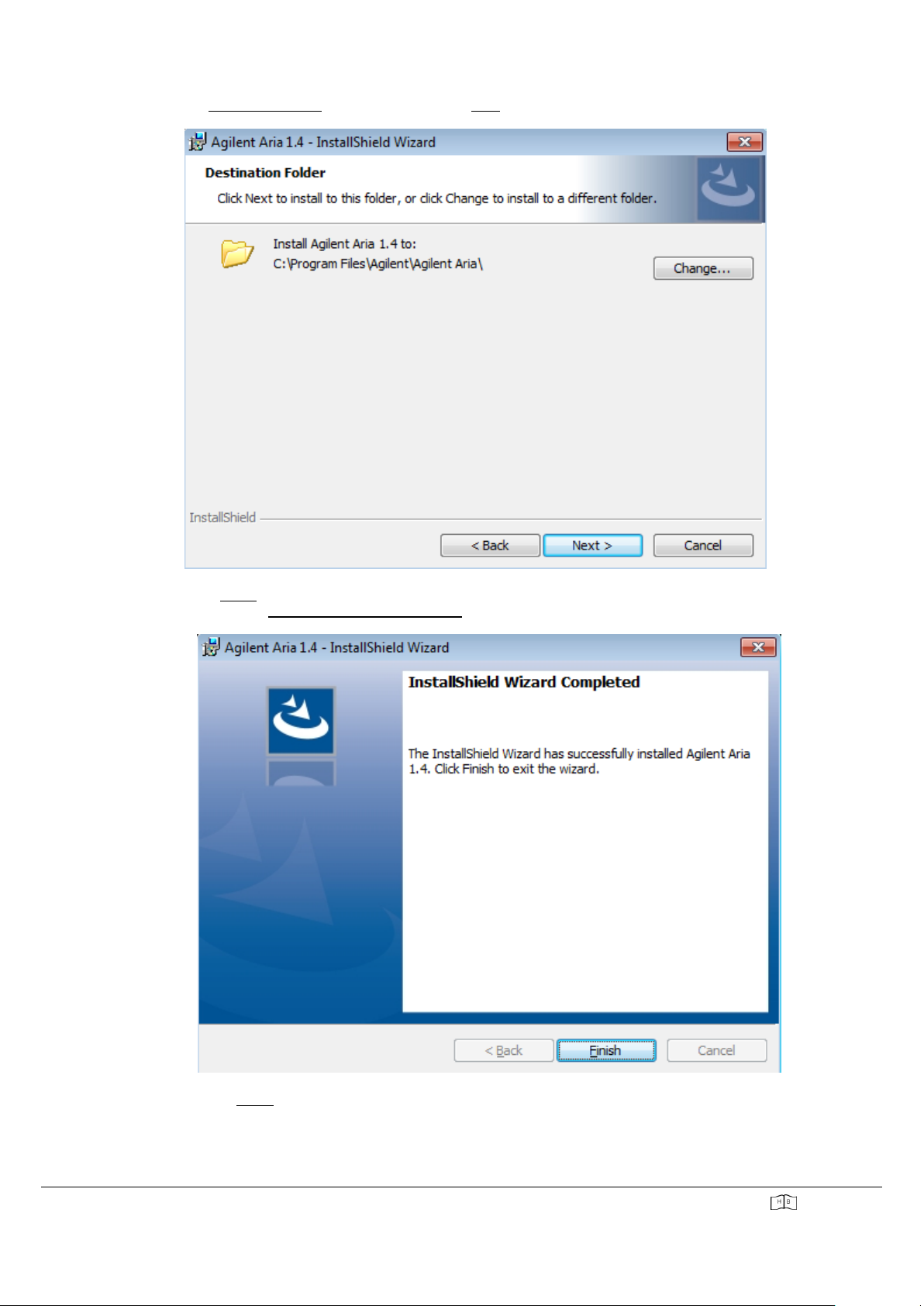
▪The "Destination Folder" window opens. Press "Next" to continue.
▪Press "Install". The wizard installs the AriaDX software in the folder shown. When the installation is
complete, the "InstallShield Wizard Completed" window opens.
▪Click “Finish”to close the wizard.

EasyPGX® qPCR instrument 96
Cat.Nr: RT800-96
13/21
2017/06
HARDWARE FUNCTIONS
LED INDICATOR
On the front of the instrument (right upper corner) there is a LED status indicator. The table below shows the color code
for this indicator:
LIGHT INDICATION
MEANING
Off
The instrument is switched off
Flashing green
The instrument is running
Fix green
The instrument is paused
Flashing red
The instrument has detected an error. Check what is shown
on the instrument display for more details
LOADING THE HEATING BLOCK
The instrument can be loaded with individual PCR tubes, strips or 96-well plates.
▪Open the instrument cover above the heating block using the handle to lift it. Open it completely.
▪Raise the heated lid by pulling the handle forward and then lifting it from the heat block.
▪Place the plate, strips or tubes on the block and check that they are correctly positioned.
▪Close the heated cover and push it back so that it locks properly.
▪Close the instrument cover and make sure it locks.
SOFTWARE FUNCTIONS
IMPORT OF TEMPLATES
“EasyPGX®qPCR instrument 96”uses the dedicated Aria DX software. The software is validated for use in
combination with the "EasyPGX®ready" kits manufactured and commercialized by Diatech Pharmacogenetics
srl. It is recommended to use only pre-set templates available on request contacting Diatech Pharmacogenetics
Import of template should only be performed the first time you use the specific kit associated with that template.
In subsequent runs it is sufficient to recall it (see paragraph "Execution of a run").
▪Save the template coupled with the kit in use to a USB stick.
▪Switch on the instrument by pressing the button on the bottom of the front panel and wait for the Home
screen to appear.
▪Insert the USB stick on the door on the instrument panel.
▪On the Home screen, press "New Experiment". The "Experiment Types" screen opens.
▪Press "Open Template". The "Template" screen opens.
▪Press "Import", select the template on the USB stick and press "OK". The template is then automatically
saved in the specific "Template" folder.
▪Remove the USB stick.
EXECUTION OF A RUN
▪Switch on the instrument by pressing the button on the bottom of the front panel and wait for the Home
screen to appear.
▪On the Home screen, press "New Experiment". The "Experiment Types" screen opens.
▪Press "Open Template". The "Template" screen opens.
▪Press on the template file associated with the kit you are using to select it and press "Open". The "Plate
Setup" screen opens.
▪Press "Sync Plate" to synchronize the acquisition channels.
▪Open the "Thermal Profile" window.
▪Press "Run Experiment" to start the run.

EasyPGX® qPCR instrument 96
Cat.Nr: RT800-96
14/21
2017/06
EXPORTING DATA
▪At the end of the session, return to the Home screen by pressing the "Home" button at the bottom left of the
display.
▪Insert the USB stick on the port on the front of the instrument.
▪Press "Saved Experiment".
▪Select the last run file from the list.
▪Press "Copy".
▪Select "USB Disk" on the left.
▪Press "Paste".
▪Return to the home screen by pressing the "Home" button at the bottom left of the display and disconnect
the USB stick.
ANALYSIS OF THE RESULTS
▪For the analysis of the results obtained with the kit of the "EasyPGX®ready" line, refer to the user manuals
that can be downloaded from the private area of the website www.diatechpharmacogenetics.com/area-
riservata

EasyPGX® qPCR instrument 96
Cat.Nr: RT800-96
15/21
2017/06
CALIBRATIONS
MOTOR CALIBRATION
The instruments are supplied fully calibrated; generally after the instrument has been received, no further calibration
cycles are required. However, in the event that the warning on the instrument's touch screen indicate the need to
calibrate the engine, press “Calibrate”, and then follow the on-screen instructions.
BACKGROUD CALIBRATION
Background calibration determines the background noise level associated with each optical module and compensates for
its level to minimize it. Each time a new optical module is installed, a message on the display shows up, requiring
calibration of its background.
When replacing the optical module installed in any slot with a different module, the touch screen prompts you to perform
the Background Calibration for the module just installed. Refer to “INSTALLATION” section of this manual for the
execution of the procedure.
TOUCH SCREEN CALIBRATION
You can calibrate the touch screen response functions to optimize sensitivity.
▪On the Home screen, press "Settings".
▪In the "Settings" screen, press "Calibrate Touch".
The next screen shows how to use a marker (+). Follow the instructions on the screen to complete the procedure.
INSTRUMENT MANTEINANCE
CLEANING THE INSTRUMENT
To clean “EasyPGX®qPCR instrument 96”, follow the steps below.
▪Use a cloth dampened with water or isopropyl alcohol to clean the outside of the instrument.
▪Avoid contact of the instrument with organic solvents or aggressive solutions.
▪Avoid the introduction of liquids into the instrument.
▪Switch off and unplug the instrument from the power supply before cleaning it.
CLEANING THE OPTICAL MODULES
To clean an optical module:
▪Remove the optical module from the slot.
a Open the lid of the moving lodging.
b Lift the lever over the optic module to pull the module out of the slot.
▪Clean the bottom surface of the optical module (the opposite side of the label) with a compressed air bottle.
Spray the air from a distance of 7-10 cm.
▪(Optional) Clean the bottom surface with a lint-cloth dampened with isopropyl alcohol or reagent grade
acetone.
▪Reinstall the optical module in the lodging. Lower the lever until it clicks.
▪Close the lodging door.
Lifting the lever of an optical module

EasyPGX® qPCR instrument 96
Cat.Nr: RT800-96
16/21
2017/06
ERROR MESSAGE / TROUBLESHOOTING
This chapter contains troubleshooting instructions in order to solve the error messages reported by the instrument.
When the instrument detects hardware or optical module problems, an error message shows up.
Error messages also appear in case of problems while running the scan application function or diagnostic tests.
When the “EasyPGX®qPCR instrument 96”detects an error, it displays a special icon at the bottom of the touch
display.
▪Press this icon.
▪In the popup menu that opens, press "Count X", where "X" matches the number of error messages to read.
▪A table opens with the following information.
oType: message type (error or warning). Warning messages are used for errors that do not prevent the
execution of experiments.
oID: Error Identification Code.
oDescription: A description of the error with its resolution instructions.
▪Press “OK” to close the table
Some error messages may refer to the diagnostic report for more details about the cause of the error.

EasyPGX® qPCR instrument 96
Cat.Nr: RT800-96
17/21
2017/06
TECHNICAL SPECIFICATIONS
Dimensions
50 cm L × 46 cm P × 42 cm H (19,7" × 18,1" × 16,5")
Weight
23 Kg
Storage condition
Temperature: 10-43 °C (50-109 °F)
Relative humidity: 10-90 % (without condensation)
Operating condition
Temperature: 20-30 °C (65-95 °F)
Relative humidity: 20-80 % (without condensation)
Maximum altitude 2000 m
Excitation source
8 LEDs for each optical module
Detection
8 photodiodes for each optical module
Channels
FAM, HEX
Power supply
100–240 VAC, 50/60 Hz, 1100 VA
Thermal system
Peltier block
Positions
96
Temperature range of the thermal system
25.0-99.9 °C
Uniformity
± 0.2.5 °C (above 60 °C); ± 0.4 °C (40-59 °C)
Maximum heating speed
> 6 °C/s
Maximum cooling speed
>2.5 °C/s
Accuracy
± 0.2 °C
Reaction volume
10 –30 µl
Data acquisition time
< 3 s

EasyPGX® qPCR instrument 96
Cat.Nr: RT800-96
18/21
2017/06
WARRANTY AND CLAUSE OF LIABILITY
WARRANTY
Diatech Pharmacogenetics srl guarantees that a new “EasyPGX®qPCR instrument 96” conforms to the stated product
specifications and is free of manufacturing defects and materials during the entire warranty period.
This limited warranty, non-transferable, is considered valid only for the buyer and can’t therefore be sold to third parties.
The warranty starts on the date of shipment of the product and is valid for twelve (12) months.
Repairs or replacement of defective parts will be guaranteed to the buyer during this period to the extent that the
instrument was used under normal operating conditions, according to the recommendations of this manual, but not for
damage caused by the customer.
If any parts prove to be defective, it will be repaired or replaced by Diatech Pharmacogenetics srl, following an inspection
carried out at the factory or onsite by an authorized technician, provided that such defect has occurred in authorized use
conditions.
EXCLUSIONS
The warranty does not apply to defects or anomalies caused by:
▪external sources such as short-circuits or incorrect voltages;
▪normal wear and tear;
▪contact with chemicals or samples that are not authorized or used inappropriately;
▪repair, modification, alteration or installation performed by persons other than Diatech Pharmacogenetics
personnel or other authorized persons;
▪handling, use or maintenance of the instrument in an inappropriate, inadequate or unauthorized manner (e.g.:
failure to follow the instructions in this manual and the user manual of Diatech Pharmacogenetics kits in which
“EasyPGX®qPCR instrument 96” is an accessory; use of the instrument in non-compliance with
environmental recommendations; use of the instrument with unapproved software, materials or other
consumable products);
▪installation of unapproved software;
▪negligence or accidents caused by the customer.
GUARANTEE LIMITATION AND REMEDIES
The warranty previously described is the unique and exclusive guarantee of Diatech Pharmacogenetics srl and the repair
or replacement of defective parts is the sole and exclusive right of remedy. No other warranties, no express or implied
warranties are provided. The implied warranties of merchantability and eligibility for particular purposes are explicitly
excluded, extended to all categories permitted by law. (Note: some jurisdictions do not contemplate the use of formulas
to deny implied warranties). With the exception of the aforementioned remedies for repair or replacement, Diatech
Pharmacogenetics srl will have no obligations or liability with regard to the instrument manufactured by Diatech
Pharmacogenetics srl if it is based on the contract, for criminal acts, for serious liability or for any other , including but not
limited to direct, indirect, incidental, consecutive, special, punitive or exemplary damages, but not limited to loss of
income or foreseen benefits or lost business and moral damages, although not foreseen or foreseeable or brought to the
attention of Diatech Pharmacogenetics srl. In no case the liability of Diatech Pharmacogenetics srl will exceed the
original purchase price of the product.
RESPONSIBILITY CLAUSE
Diatech Pharmacogenetics srl will be exonerated from any liability during the warranty period if repairs or modifications
were made by persons not belonging to its staff, except when the Company has given its written approval to perform
repairs or modifications.
All materials will only be replaced during the original warranty period and in no case beyond the date of expiration of the
original warranty, unless authorized by a Company Manager. Read-only devices, accessories, interface devices, and
related software will be covered by the warranty only for the period provided by the original Manufacturer of these
products. Any declarations and warranties issued by anyone, including representatives of Diatech Pharmacogenetics srl,
which are inconsistent or conflicting with this warranty, will not be binding for the Company unless agreed in writing and
approved by a Manager of Diatech Pharmacogenetics srl.

EasyPGX® qPCR instrument 96
Cat.Nr: RT800-96
19/21
2017/06
APPENDIX A: ELECTRIC AND ELECTRONIC WASTE DISPOSAL DIRECTIVE (WEEE)
The following section provide information regarding the disposal of electrical and electronic equipment - WEEE by end
users.
The product described in this user manual is an equipment covered by the Directive 2012/19/EU and therefore on the
instrument is applied the following symbol (cross on a waste bin) indicating that this product can’t be disposed of as
urban waste, but must be brought to an approved waste treatment facility or at an authorized recycling facility, in
accordance with local laws and regulations
Recycling of WEEE when disposed of will ensure the conservation of natural resources and ensure that the product is
recycled in accordance with the protection of human health and environment.
For this reason, Diatech Pharmacogenetics has implemented, in compliance with the provisions of the law in force, a
specific procedure for the return of electrical and electronic equipment (EEE) with its own trademark that have become
unusable, or for broken spare parts.
General conditions for products return
In case of request by the Customer to return to Diatech Pharmacogenetics the discarded product for recycling / disposal
in compliance with current legislation on WEEE (Waste Electrical and Electronic Equipment) the following conditions are
applied:
▪Unless otherwise agreed, only products sold within the European Union can be returned.
▪Returned products must not be contaminated with substances dangerous for people and / or environment. The end
user of the products must certify this with a specific Declaration of Decontamination, which must be handed over to
the carrier.
▪The End User is responsible for the deletion and / or removal of any sensitive data contained in the product being
returned.
▪Shipping costs will be charged to the Customer unless otherwise agreed with Diatech Pharmacogenetics.
▪Packaging conditions - All products must be properly packed to ensure their protection during transport.
▪Return costs - Diatech Pharmacogenetics will bear all costs related to the treatment, recycling and reclamation of
the product being returned, except for shipping costs and for decontamination required prior to packaging as per
previous points.
▪At the time of collection, on the established day and time, a customer representative will have to deliver the product
ready for collection, accompanied by a delivery note with "DISPOSAL" reason. The Customer will be responsible for
the costs in case of delivering wrong products.
▪Assignment - The End User transfers to Diatech Pharmacogenetics all rights regarding the title, treatment, recovery,
and recycling of the product, free from any encumbrance. The Customer guarantees to have the full availability and
the title to return the product at Diatech Pharmacogenetics.
▪Diatech Pharmacogenetics is not responsible for products returned by mistake and does not guarantee the return of
the same to the Customer. If these products can be returned, the customer must bear the shipping costs and any
additional administrative costs.
For all necessary agreements and for the Declaration of Decontamination, or for any other clarification, contact Diatech

EasyPGX® qPCR instrument 96
Cat.Nr: RT800-96
20/21
2017/06
OPERATOR NOTES
Table of contents
Popular Laboratory Equipment manuals by other brands

VWR
VWR avantor STAR-BEATER Short instruction manual

Steris
Steris Basil 4700 Maintenance manual

Resodyn
Resodyn Manual Vacuum Control Module Installation and user manual

Monmouth Scientific
Monmouth Scientific Circulaire FB250 operating & maintenance manual

Metrohm
Metrohm ProfIC Vario 6 Anion manual

REITEL
REITEL VACURET DIGIT operating instructions

HAVER & BOECKER
HAVER & BOECKER EML 200 Pure operating instructions

Heidolph
Heidolph Hei-MIX Titramax 100 operating instructions

Dynamica
Dynamica VELOCITY 14R Pro instruction manual

Milestone
Milestone MAXI-44 user manual

Azure Biosystems
Azure Biosystems chemiSOLO user manual

3M
3M Attest 390 Quick reference guide