4
IMPORTANTINFORMATION
1. INSTRUCTIONS
1.1 EU -Labelling
The product carries EU labelling in
accordance with the Council’s guideline on
medicinal products (EU 93/42) and complies
with the basic requirements contained in
appendix 1 of this guideline.
1.2 Guidelines
• The requirement for electromagnetic
capability related to electric medical
appliances has been complied with.
1.3 General Instructions
• The instructions for mounting and using the
appliance are an integral part of the
machine. They must always be stored
close to the machine. These instructions
must be strictly complied with so that the
machine can be used and correctly
operated in accordance with the relevant
regulations. New employees must be
acquainted with the instructions for
mounting and using the machine
• So that the machine may be operated safely
and in a trouble-free manner, original parts
must be used. In addition, only
accessories mentioned in the technical
documentation or expressly released by
Dürr Dental for this purpose may be used.
• No warranty claims shall be made for
damage caused by the use of accessories
or consumables manufactured by external
companies. Dürr Dental can give no
warranty for the safe operating and
functioning of the machine.
• With regard to safety, reliability and
functionality, Dürr Dental shall only be
responsible if the machine has been
mounted, assembled, altered, enlarged or
repaired by Dürr Dental or by a specialist
authorised by Dürr Dental, and if the
machine was used and operated in
accordance with the instructions for
mounting and use.
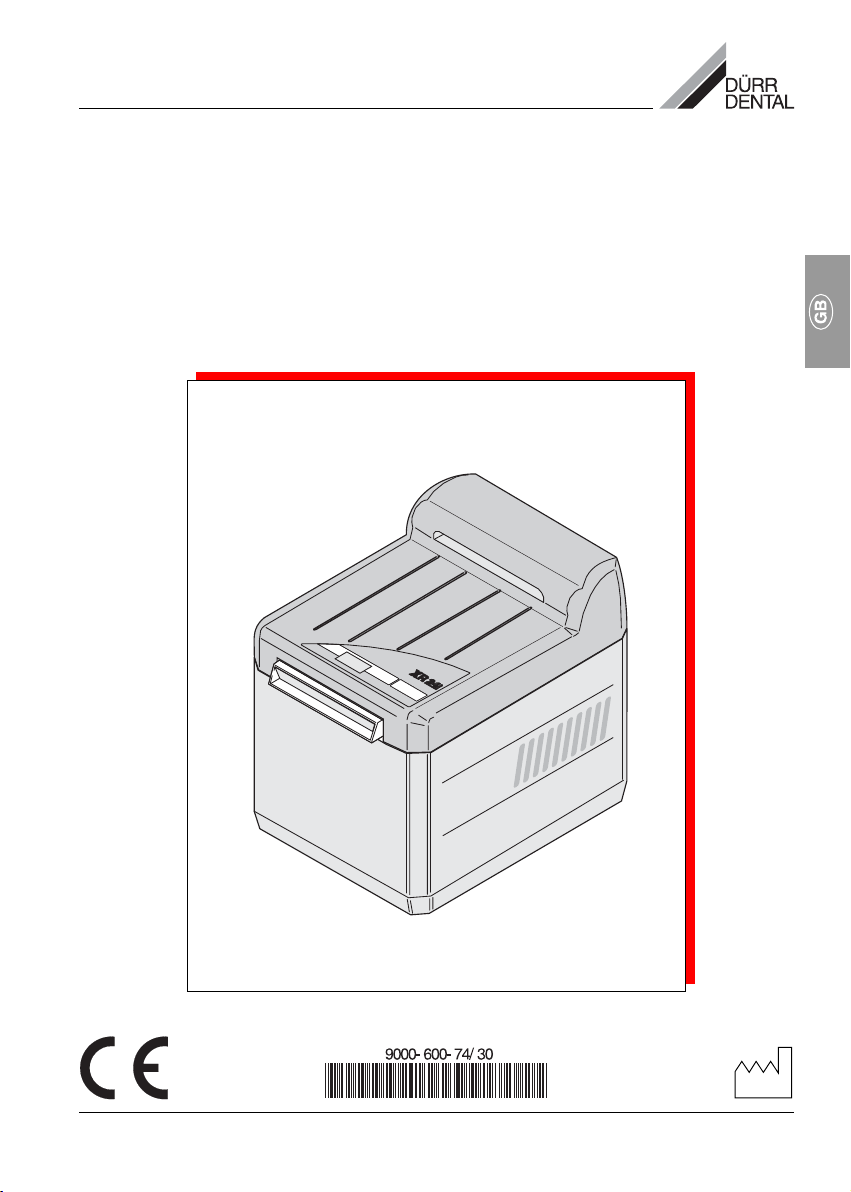
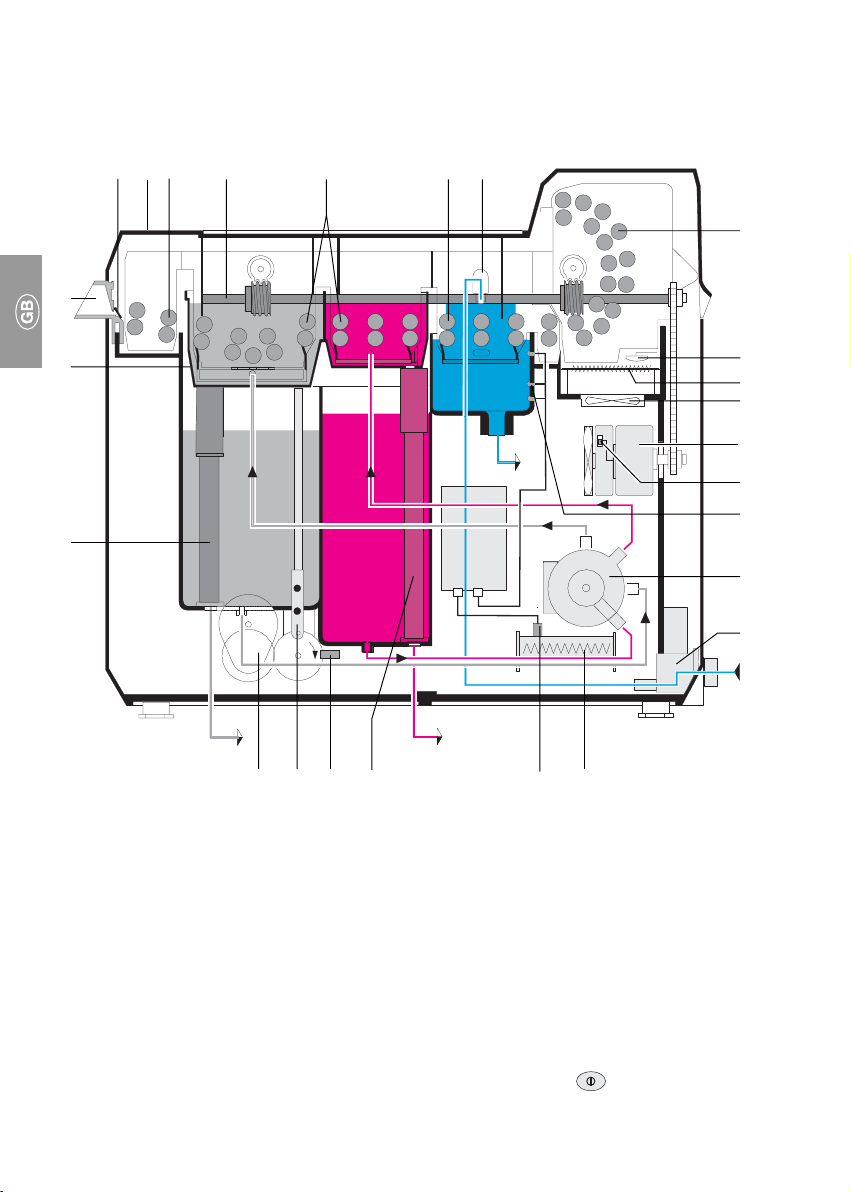
• The XR 24 PRO developing appliance fulfils
the basic norms on safety and technology.
All rights of protection are reserved for the
circuits, procedures, names, software
programs and appliances mentioned in the
documentation.
• The technical documentation, or parts
thereof, may only be reprinted with the prior
written permission of Dürr Dental.
1.4 General Safety Instructions
The appliance was developed and designed
in such a way that dangers associated with
use in accordance with the relevant
regulations are largely ruled out. However,
we are obliged to list the following safety
measures so that residual dangers can also
be ruled out..
• When the machine is being operated, the
laws and regulations applying in the place
of use must be complied with. It is illegal to
reconstruct or alter the machine in any way;
contravention of this regulation shall lead to
expiry of the permit. If an altered machine
is operated, the operator shall be liable to
prosecution. When the machine is being
operated, the laws and regulations applying
in the place of use must be complied with.
It is illegal to reconstruct or alter the
machine in any way; contravention of this
regulation shall lead to expiry of the permit.
If an altered machine is operated, the
operator shall be liable to prosecution.
• The original packaging must be stored in
case the appliance has to be returned. The
packaging must be kept out of the reach of
children. Only the original packaging can
guarantee that the machine is protected in
the optimum manner while it is being
transported. If the machine has to be
returned during the warranty term, then Dürr
Dental shall not be liable for any damage
which occurred as a result of faulty
packaging while the machine was being
transported.
• The machine complies with the relevant EU
guidelines and may only be used by
qualified persons who are capable of
operating it in the appropriate manner.