echosens FibroScan 502 touch User manual

FibroSca
“EA
FibroScan”
502
TOUCH
User
Manual
CE
0459
E300M017.4
—
Version
4
—
03/2017
(software
version
C
3.1)

ETS
FIBROSCAN
502
TOUCH
USER
MANUAL
FibroScan
2
03/2017
-
ECHOSENSTM
AND
FIBROSCAN®
ARE
TRADEMARKS
©
COPYRIGHT
ECHOSENS
-
ALL
RIGHTS
RESERVED
E300M017.4

FibroScan
FIBROSCAN
502
TOUCH
USER
MANUAL
BET
1.
11
12
2.
2.1
2:2;
2.3.
2.4.
2.5.
2.6.
2.7.
2.8
2.9
3.
3.1
3.2
33
34
3.5
3.6
37
4.
4.1.
4.2.
4.3.
4.4.
4.5.
4.6.
4.7.
5.
5.1
5.2
5.3
5.4
5.5
6.
TABLE
OF
CONTENTS
PURPOSE
OF
THE
USER
MANUAL........
ner
e
onen
6
.
Symbols
used
in
the
Manual
ee
7
.
Property
and
copyright...
GR
R
R
R
7
ΜΙΑΒΝΙΝΟΒ.
ΟΗΕ
εν
A
σώος
8
;
GerisralıInformalleiN
siii
με με
VAE
8
[τι
ο
Ες
anaya
NEA
8
Electromagnetic
safety
.
Using
the
device.
Deleting
measurements.
Switching
off
the
device
Cleaning
and
maintenance...
nenene
nenn
9
.
Cleaning
.
Interpreting
the
results
MISCELLANEOUS
INFORMATION
oe
11
.
Guarantee
.
Liability...
.
Essential
performance
characteristics...
11
.
Product
life...
sens
.
Reverse
engineering
.
Registered
trademark:
.
Patented
technology..
INDICATIONS
AND
PRECAUTIONS
FOR
USE
Intended
use.
Indications
for
use
Probeandexaminationselectioncriteria.......................................
16
Precautions
for
use...
17
User
training
....
Electrical
safety
.
Maintenance-related
safely
sussa
SALES
18
EXTERNAL
PRESENTATION
.
Hardware
supplied
.
ACCessories
лилии
иная
.
Front
view
.
Rear
view
.
Description
of
probes.
SOFTWARE
INTERFACE...........................rcrrrrcereeeeieire
ricer
rece
AEG
REDDE
26
E300M017.4
03/2017
-
ECHOSENSTM
AND
FIBROSCAN®
ARE
TRADEMARKS
©
COPYRIGHT
ECHOSENS
-
ALL
RIGHTS
RESERVED
3

ETS
FIBROSCAN
502
TOUCH
USER
MANUAL
FibroScan
6.1.
6.2.
6.3.
6.4.
6.5.
6.6.
6.7.
7.
STANDBY
AND
SHUTTING
DOWN
THE
DEVICE.
τα,
Tdi
8.
CLEANING,
MAINTENANCE
AND
REPAIRS
8.1.
8.2.
8.3.
Home:SCresn
İŞ
YY
26
Usingthekeypad...........................................
erene
27
Login
Window...
sise
27
Status
bar
The
patient
record
screen
Acquisition
screen
6.6.1.
Patient
data
6.6.2.
Ultrasound
images...
6.6.3.
Pressure
indicator
6.6.4.
Shear
wave
propagation
map
6.6.5.
Counters:
Valid
and
invalid
measurements
......................
n
35
6.6.6.
Shear
wave
speed
results
area
6.6.7.
Stiffness
results
area
6.6.8.
CAP
result
zone
(option)
6.6.9.
Deleting
measurementS
nenene
6:6.10-Adding'a
GomMmMEN
tisser
6.6.11.Integrate
the
measurement
conditions
6.6.12.
Message
area...
6.6.13.
Examination
type
selection
area.
6.6.14.View
and
print
theresultoftheexamination.............................................
43
Management
of
patient
file
archives..
6.7.1.
Advanced
file
search
6.7.2.
Selectand
view
a
patient
file
6.7.3.
Details'of
the
examination...
ο
ο
P
ARE
RnR
nen
Er
Between
sessions
τε
At
the
end
of
the
day
sisi
ob
eee
47
Cleaning
8.1.1.
Cleaning
the
device
(painted,
metallic,
glass,
or
plastic
surfaces
and
screen)
8.1.2.
Cleaning
the
probe
(housing,
cable
and
transducer)
8.1.3.
Recommended
cleaning
products
ce
8.1.4.
Recommended
decontamination
solutions.................................................
Calibrating
the
probe
..
Troubleshooting
9.
CONFIGURING
THE
FIBROSCAN
ie
52
9.1.
9.2.
9.3.
9.4.
9.5.
9.6.
9.7.
9.8.
9.9.
Entering
configuration
mode
Localization
Tab
Institution
tab
....
Printer
tab…
せ
em
Data
Tab
User
Tab
Connectivity
Tal
[ЕЕ
ος
ο
ο
ο
ο ο
ener
55
System
4аБ........
ο
ο
ο
ο
ο
55
4
03/2017
-
ECHOSENSTM
AND
FIBROSCAN®
ARE
TRADEMARKS
©
COPYRIGHT
ECHOSENS
-
ALL
RIGHTS
RESERVED
E300M017.4

FibroScan
FIBROSCAN
502
TOUCH
USER
MANUAL
ETE]
E
IMM
rr
SA
55
GOD
LOGS
usas
ar
ara
σος
56
A
ο
ο
ο
σος
ο
v
ire
eS
56
A
ο
ο
ο
ο
ο
57
10.
syMBoLs
oNTHEDEVICE
neon
eee
에나
58
10.1.
Connectors
ss
사
아
아아아
58
10.2.
Warnings
10.3.
Marking
and
electrical
safety
11.
TECHNICAL
CHARACTERISTICS
11.1.
Characteristics
of
the
device
..
11.1.1.
Computer
characteristics
.
11.1.2.
Metrological
performance
11.1.3.
Electrical
characteristics
iii
ent
11.1.4.
Mechanical
characteristics
11.1.5.
Environmental
characteristics
11.1.6.
Additional
information
προ
ο
ο
ο
ο
ο
ο
τον
ο
ο
가
64
12.
REGULATIONS
rra
65
12.1.
Electromagnetic
emissions
12.2.
Electromagnetic
immunity
(1)
12.3.
Electromagnetic
immunity
(2)
12.4.
Recommended
separation distances
E300M017.4
03/2017
-
ECHOSENSTM
AND
FIBROSCANO
ARE
TRADEMARKS
€
COPYRIGHT
ECHOSENS
-
ALL
RIGHTS
RESERVED
5

ETS
FIBROSCAN
502
TOUCH
USER
MANUAL
FibroScan
1.
PURPOSE
OF
THE
USER
MANUAL
This
User
Manual
has
no
contractual
value
whatsoever
and
under
no
circumstances
may
Echosens
be
held
responsible
on
the
basis
of
the
information
contained
in
this
Manual.
This
User
Manual
details,
on
the
one
hand,
all
of
the
information
required
for
the
implementation,
use
and
maintenance
of
the
FibroScan
device
and,
on
the
other
hand,
the
list
of
information
displayed.
Thus,
after
carefully
reading
the
manual,
operators
will
be
able
to:
=
connect
peripheral
elements
(power
cable,
USB
devices,
probes)
and
power
up
the
FibroScan
device,
m
configure
the
device,
в
navigate
the
device's
user
interface,
m
perform
basic
maintenance.
Echosens
publishes
this
manual
"as
is",
without
guarantees
of
any
nature,
whether
explicit
or
implicit,
including,
but
not
limited
to
implicit
guarantees
or
merchant
conditions,
or
adaptation
for
specific
use
in
view
of
providing
simple
and
accurate
information.
Consequently,
Echosens
SA
cannot
accept
any
responsibility
for
any
incorrect
interpretation
of
the
Manual.
Although
all
efforts
have
been
made
to
offer
a
manual
that
is
as
accurate
as
possible,
this
manual
may
nevertheless
contain
some
technical
inaccuracies
and/or
typographical
errors.
Echosens
cannot,
under
any
circumstances,
be
held
responsible
for
any
loss
of
profit,
loss
of
business,
data
loss,
business
interruption,
or
for
any
indirect,
specific,
accidental
or
consecutive
damages
of
any
type.
In
the
event
of
damages
arising
from
a
defect
(imperfection)
or
error
contained
in
this
User
Manual,
Echosens
undertakes
to
send
the
physician,
as
rapidly
as
possible,
a
hard
copy
or
electronic
document
containing
all
corrections
made
to
this
manual.
This
manual
is
updated
on
a
regular
basis.
The
most
recent
version
of
this
manual
is
available
from
Echosens
on
request.
Should
any
major
modifications
be
made
to
the
manual,
however,
Echosens
undertakes
to
send
the
physician,
as
rapidly
as
possible,
a
new
copy
of
the
manual
in
hard
copy
or
electronic
format.
Note
that
this
does
not
involve
updating
the
hardware
and/
or
software
in
your
possession.
The
product
owner
must
keep
this
manual
for
as
long
as
the
product
is
used.
This
manual
contains
a
chapter
for
troubleshooting
the
most
commonly
encountered
problems.
Any
information
or
modification
requests
pertaining
to
this
manual
should
be
sent
to:
Echosens,
30
place
d'ltalie,
75013
Paris,
France.
6
03/2017
-
ECHOSENSTM
AND
FIBROSCAN®
ARE
TRADEMARKS
©
COPYRIGHT
ECHOSENS
-
ALL
RIGHTS
RESERVED
E300M017.4

FibroScan
FIBROSCAN
502
TOUCH
USER
MANUAL
EET
1.1.
SYMBOLS
USED
IN
THE
MANUAL
个
This
symbol
means:
CAUTION
Warning:
See
the
instructions
before
using
the
medical
device.
Instructions
preceded
by
this
symbol
may
cause
injuries
or
damage
the
medical
device
and
installation
if
not
correctly
followed.
This
symbol
means:
INFORMATION
Additional
information
with
no
impact
on
device
use.
1.2.
PROPERTY
AND
COPYRIGHT
All
manuals
and
documents
of
all
kinds
are
the
property
of
Echosens
and
are
protected
by
copyright,
all
rights
reserved.
Your
right
to
copy
this
documentation
is
limited
to
legal
copyright.
These
manuals
cannot
be
distributed,
translated
or
reproduced,
either
in
whole
or
in
part,
in
any
manner
or
in
any
form,
without
prior
written
consent
from
Echosens.
Hence,
the
reproduction,
adaptation
or
translation
of
this
manual
without
prior
written
consent
is
prohibited,
within
the
limits
provided
by
copyright
law.
Copyright
©
—
09/2015
—
03/2017
—
Echosens
—
All
rights
reserved.
E300M017.4
03/2017
-
ECHOSENSTM
AND
FIBROSCAN®
ARE
TRADEMARKS
©
COPYRIGHT
ECHOSENS
-
ALL
RIGHTS
RESERVED
u

#'LLONO0C3
CINYISIY
SLHONI
TIY
-
SN3SOH93
1HOIMAdOD
©
SHAYWAOYVUL
SHY
GNVOISONBIS
ONY
WLSN3SOH93
-
4102/00
8
*SoUBqJn]SID
9119u5BUJOJ198I9
Sg1EJguSb
1641
94140641
6
JBSU
JO
UOdn
DeoBId
ueuW
solAap
ugoSoJql4
aul
6ulsn
ploAV
(OWA)
Auiquedwos
эцаибешолове
40
SUJJ9]
ul
sougllduuoo
-uou
e
asnes
Áew
IlenuelN
Jasn
sul
ul
paulosds
lou
seuossaooe
jo
asn
ayl
ALAAVS
ODILINOVINOYLI
313
“EZ
‘abewep
a|gissanai
Buisneo
‘Jeaylano
Aew
jueudinbe
oiuosoeje
au}
‘ale
Áay)
y]
“pajon.]sqo
jou
aye
squan
au}
Jeu}
9JnS
ayeW
<
“spJepuejs
ΛΙΘ/85
Ulm
juei]duoo
}ax90s
E
ol
paloauuoo
SI
wa]sAs
au}
Ji
0981461606
80
시
40
4620
uonesedo
Buipunoif
1981109
“Wa]sAs
au}
0]
pajoauuoo
ag
Jou
Jsnw
|enuejy
Jesn
ay}
ul
palioads
jou
sped
AUV
‘L-06609
931
PIBPUEYS
0}
Buipiooe
peuiues
aq
lsnu
jndjnogndu!
sjeubis
o]
pajosuuos
sjessuduad
м
"SUJgIdOJd
UOIO9UUO9
USA8」d
ol
alqlsseooe
Allsea
Θα
1snlu
pug
эомер
Bunejos!
ue se
asn
Jo,
papuajul
SI
jeY00S
alqeo
JaNod
su
上
(4
4
414
“BOIA9P
OU]
ol
peloeuuoo
sq lou
|snty
Spes|
uolsus]xe
pue
sisjdepe
}9x90S-IININ
“Ájuo
Alddns
samod
pepunol
ue
0)
pejosuuoo
aq
JsNW
891A8P
SIL}
‘HIOUS
91J98[8
JO
YSU
BU}
PIOAE
OL
AL33VS
IVIOINLIATA
‘e
|
"UBIDISÁUC
€
JO
Japlo
au}
UO
JO
Аа
эез
о}
SOIASD
SIU1
S1OU1SSJ
Me]
¡ejapay
:uonneg
y
NOILLVINHOANI
ЕЕ
上
<
SONINAVM
と
ues
Souq
TvnNvW
Y3SN
HONOL
Zos
NvOsOUsIs
SE)

FibroScan
FIBROSCAN
502
TOUCH
USER
MANUAL
TRUE]
concerning
electromagnetic
compatibility
(EMC).
It
must
be
installed
and
set
up
according
to
the
EMC
information
given
in
this
manual.
A
The
FibroScan
502
TOUCH
device
requires
special
precautions
to
be
taken
2.4.
USING
THE
DEVICE
个
Do
not
push
or
lean
on
the top
of
the
FibroScan
device.
To
avoid
tipping
the
FibroScan
when
moving
it,
move
it
slowly
sideways
and
steer
it
firmly
by
holding
the
decorative
rods
on
the front
and
back
of
the
device.
To
avoid
tipping
the
FibroScan
when
lowering
it
down
a
step,
the
operator
must
go
in
front
of
the
device
and guide
it
on
the
way
down.
2.5.
DELETING
MEASUREMENTS
All
measurements
taken
before
the
one
chosen
for
deletion
will
be
eliminated
from
the
examination
after
confirmation.
2.6.
SWITCHING
OFF
THE
DEVICE
Never
switch
the
device
off
during
an
examination
or
whilst
in
configuration
mode.
A
Never
disconnect
the
main
power
supply
when
the
device
is
switched
on.
Failure
to
comply
with
these
instructions
may
cause
a
malfunction
of
the
device
and/or
loss
of
data.
2.7.
CLEANING
AND
MAINTENANCE
These
maintenance
operations
must
not
be
performed
by
a
third
party
other
than
a
technician
authorized
by
Echosens.
The
opening
or
modification
of
the
device
by
any
person
other
than
an
authorized
Echosens
technician
is
strictly
prohibited.
No
CD
ROMs
or
DVD
ROMs
other
than
those
provided
by
Echosens
should
be
inserted
into
the drive.
The probe
must
be
calibrated
periodically.
Beyond
this
period,
the
manufacturer
no
longer
guarantees
the
performance
characteristics
of
the
probe.
2.8.
CLEANING
To
prevent
electric
shock,
switch
off
the
device
and
disconnect
it
from
the
power
supply
before
cleaning.
E300M017.4
03/2017
-
ECHOSENSTM
AND
FIBROSCAN®
ARE
TRADEMARKS
©
COPYRIGHT
ECHOSENS
-
ALL
RIGHTS
RESERVED
o

EE
FIBROSCAN
502
TOUCH
USER
MANUAL
FibroScan
2.9.
INTERPRETING
THE
RESULT:
Results
must
only
be
interpreted
by
a
physician
specializing
in
liver
diseases,
who
is
aware
of
the
patient's
pathology
and
clinical
context.
10
03/2017
-
ECHOSENSTM
AND
FIBROSCAN®
ARE
TRADEMARKS
©
COPYRIGHT
ECHOSENS
-
ALL
RIGHTS
RESERVED
E300M017.4

FibroScan
FIBROSCAN
502
TOUCH
USER
MANUAL
[ETUE
3.
MISCELLANEOUS
INFORMATION
3.1.
GUARANTEE
The
terms
of
guarantee
are
stated
in
the
Echosens
terms
of
sale
documents.
For
any
requests,
Echosens
is
at
the
disposal
of
the
physician
and
their
assistants
and
shall,
if
necessary,
pass
the
aforementioned
request
on
to
the
competent
local
representative.
3.2.
LIABILITY
The
information
displayed
on
the
FibroScan
screen
is
the
result
of
complex
calculations
performed
by the
software
application
built
into
the
FibroScan.
These
results
are
then
interpreted
by
the
physician
in
charge.
Under
no
circumstances,
and
even
if
Echosens
had
been
notified,
could
Echosens
be
held
responsible
for
the
incorrect
interpretation
of
these
results;
Echosens'
liability
being
limited
to
making
the
measurements,
displaying
them
and
printing
them
via
the
FibroScan.
The
data
from
each
examination
are
saved
on the
device's
hard
disk.
The
user
is
responsible
for
saving
the
data
on
a
regular
basis.
Echosens
cannot
under
any
circumstances
be
held
liable
for
the
partial
or
total
loss
of
FibroScan
data.
3.3.
ESSENTIAL
PERFORMANCE
CHARACTERISTICS
The
essential
performance
of
the
FibroScan
device
is
the
medically-usable
measurement
of
liver
stiffness.
The
measurement
is
taken
at
50
Hz
shear
wave
frequency.
When
the
optimum
conditions,
particularly
relating
to
electromagnetic
disturbance,
cannot
be
obtained,
the
FibroScan
device
does
not
display
any
measurements,
but
displays
a
warning
message
to
notify
the
user
that
measurements
are
not
possible.
When
a
stiffness
or
CAP
(Controlled
Attenuation
Parameter)
value
is
displayed
by
FibroScan,
this
value
is
correct
within
the
range
of
error
specified
by
Echosens.
Free
from
the
production
of
unintended
or
excessive
transducer
assembly
surface
temperature.
3.4.
PRODUCT
LIFE
Echosens
guarantees
the
specifications
and
performance
characteristics
of
the
FibroScan
device
for
seven
years,
provided
that
all
necessary
precautions
for
use
and
maintenance
have
been
taken
in
accordance
with
the
recommendations
of
the
user
manuals
provided.
E300M017.4
03/2017
-
ECHOSENSTM
AND
FIBROSCAN®
ARE
TRADEMARKS
©
COPYRIGHT
ECHOSENS
-
ALL
RIGHTS
RESERVED
11

EE
FIBROSCAN
502
TOUCH
USER
MANUAL
FibroScan
3.5.
REVERSE
ENGINEERING
The
software
license
is
individual
and
cannot,
under
any
circumstances,
be
transferred
in
any
manner
to
a
third
party.
This
software
cannot
be
distributed,
reproduced,
translated,
disassembled,
decompiled,
analyzed,
modified,
incorporated
or
combined
with
another
software
application,
with
the
exception
of
cases
allowed
by
law.
Resale
of
the
software
built
into
the
FibroScan
is
prohibited.
3.6.
REGISTERED
TRADEMARKS
Echosens
and
FibroScan
are
registered
trademarks
of
Echosens.
Microsoft
Excel
and
Windows
Embedded
are
registered
trademarks
of
Microsoft
Corporation
in
the
United
States
and
other
countries.
3.7.
PATENTED
TECHNOLOGY
FibroScan
is
covered
by
one
or
more
patents,
both
in
the
United
States
and
in
other
countries.
Patents:
www.echosens.com/patents
12
03/2017
-
ECHOSENSTM
AND
FIBROSCAN®
ARE
TRADEMARKS
©
COPYRIGHT
ECHOSENS
-
ALL
RIGHTS
RESERVED
E300M017.4

FibroScan
FIBROSCAN
502
TOUCH
USER
MANUAL
ΓΠΕΤΕ]
4.
INDICATIONS
AND
PRECAUTIONS
FOR
USE
4.1.
INTENDED
USE
The
FibroScan
and
its
probes
form
an
active,
non-implantable
medical
device
using
ultrasound.
This
device
is
designed
to
be
used
in
a
physician's
office.
The
FibroScan
system
is
designed
to
provide
50
Hz
shear
wave
speed
measurements
and
estimates
of
tissue
stiffness
as
well
as
a
3.5
MHz
ultrasound
coefficient
for
the
Attenuation
Parameter
(CAP:
Controlled
Attenuation
Parameter)
in
internal
structures
of
the
body.
The
FibroScan
device
is
based
on
Vibration-Controlled Transient
Elastography
(VCTE
M).
The
FibroScan
probe
comprises
a
single-element
ultrasound
transducer
mounted
on
the
shaft
of
the
electrodynamic
transducer.
This
transducer
generates
a
transient
vibration,
which
in
turn
generates
an
elastic
shear
wave.
This
wave
propagates
through
the
skin,
the
subcutaneous
tissues,
and
then
the
liver.
During
shear
wave
propagation,
the
ultrasound
transducer
performs
a
series
of
ultrasound
acquisitions
(emission/reception)
to
measure
the
speed
of
shear
wave
propagation
(Vs)
in
m/s.
This
measurement
corresponds
to
the
spatial
and
temporal
average
speed
of
propagation
of
the
shear
wave
through
the
liver
region
of
interest,
which
can
be
approximated
by
a
cylinder
with
a
diameter
of
1
cm
and
a
length
of
4
cm
(corresponding
to
approx.
3
cm’).
Additionally,
assuming
that
the
liver
is
a
pure
elastic,
linear
and
isotropic
medium,
the
device
converts
shear
wave
speed
Vs
into
equivalent
stiffness
E
in
kPa
using
the
equation
E
=
3
xp
x
Vs?
with
p
the
medium
density
assumed
to
be
1000
kg/m*.
The
values
for
shear
wave
speed
and
equivalent
stiffness
(or
Young’s
modulus)
are
relative
indexes
intended
only
for
the
purpose
of
comparison
with
other
measurements
performed
using
FibroScan
devices.
Concomitantly,
the
ultrasound
acquisitions
are
used
to
assess
the
Controlled
Attenuation
Parameter
(CAP).
Ultrasound
attenuation
corresponds
to
the loss
of
energy
as
ultrasound
propagates
through
the
medium.
Due
to
attenuation,
the
intensity
of
emitted
ultrasound
(lo)
decreases
exponentially
with
depth
(z):
Iz
=
lo
x
exp
(-
a(f)
x
z)
where
Iz
is
the
ultrasound
intensity
at
depth
z
and
a
is
the
frequency-
(f)
dependent
attenuation
coefficient.
Ultrasound
attenuation
depends
principally
on
(i)
the
ultrasound frequency,
and
(ii)
the
properties
of
the
medium
of
propagation.
CAP
assesses
the
value
of
a
at
the
frequency
f
=
3.5
MHz
and
is
expressed
in
dB/m.
Absolute
values
for
these
measurements
may
vary
between
measurement
devices
produced
by
different
manufacturers.
E300M017.4
03/2017
-
ECHOSENSTM
AND
FIBROSCAN®
ARE
TRADEMARKS
©
COPYRIGHT
ECHOSENS
-
ALL
RIGHTS
RESERVED
13

[ETS
FIBROSCAN
502
TOUCH
USER
MANUAL
FibroScan
4.2.
INDICATIONS
FOR
USE
The
FibroScan
is
indicated
for
non-invasive
measurement
in
the
liver
of
50
Hz
shear
wave
speed
and
estimates
of
stiffness
as
well
as
determining
a
3.5
MHz
ultrasound
coefficient
for
the
Attenuation
Parameter
(CAP:
Controlled
Attenuation
Parameter).
The
shear
wave
speed,
stiffness,
and
CAP
may
be
used
as
an
aid
in
the
clinical
management
of
adult
patients
with
liver
disease.
Shear
wave
speed
and
stiffness
may
be
used
as
an
aid
in
the
clinical
management
of
pediatric
patients
with
liver
disease.
How
to
use
a
probe:
A:
Ultrasound
transducer.
B:
Electrodynamic
transducer.
C:
Liver.
The
values
obtained
must
be
interpreted
by
a
physician
experienced
in
dealing
with
liver
disease,
taking
into
account
the
complete
medical
record
of
the
patient
and
the
potential
presence
of
different
factors
known
to
influence
liver
shear
wave
speed
or
equivalent
stiffness
and
CAP.
Based
on the
existing
literature,
the
following
table
1
provides
a
list
of
parameters
known
to
increase
liver
shear
wave
speed
and
stiffness.
Table
1:
Parameters
related
to
shear
wave
speed
and
stiffness.
Parameter
Reference
Liver
fibrosis,
cirrhosis
[1-9]
Acute
hepatitis,
inflammation,
ALT
flares
|
[10-13]
Portal
pressure,
central
venous
pressure
| [14-16]
Extra-hepatic
cholestasis
[17]
Congestion
(heart
failure)
[18]
Meal
intake
[19]
Amyloidosis
[20-22]
For
shear
wave
speed
measurement,
the
intra-
and
inter-operator
agreement
has
been
assessed
in
a
cohort
of
200
adult
patients
with
chronic
liver
disease
of
various
etiologies
[23].
The
intra-class
correlation
coefficient
was
0.98 both
within
and
between
operators.
Moreover,
in
a
cohort
of
31
NASH
children,
a
0.96
inter-operator
intra-class
correlation
coefficient
was
found
[24].
This
demonstrates
that
intra-operator
reproducibility
is
excellent
for
shear
wave
speed
and
stiffness
measurements,
and
that
changing
the
operator
does
not
increase
measurement
variability
in
either
adults
or
children.
[1]
Friedrich-Rust,
M.,
et
al.,
Performance
of
transient
elastography
for
the
staging
of
liver
fibrosis:
a
meta-analysis.
Gastroenterology,
2008.
134(4):
p.
960-74.
14
03/2017
-
ECHOSENSTM
AND
FIBROSCAN®
ARE
TRADEMARKS
©
COPYRIGHT
ECHOSENS
-
ALL
RIGHTS
RESERVED
E300M017.4

FibroScan
FIBROSCAN
502
TOUCH
USER
MANUAL
[RUE]
[2]
Musso,
G.,
et
al.,
Meta-analysis:
Natural
history
of
non-alcoholic
fatty
liver
disease
(NAFLD)
and
diagnostic
accuracy
of
non-invasive
tests
for
liver
disease
severity.
Annals
of
Medicine,
2011.
43(8):
p.
617-49.
[3]
Shaheen,
et
al.,
FibroTest
and
FibroScan
for
the
Prediction
of
Hepatitis
C-Related
Fibrosis:
A
Systematic
Review
of
Diagnostic
Test
Accuracy.
American
Journal
of
Gastroenterology,
2007:
p.
1-12.
[4]
Shi,
K.Q.,
et
al.,
Transient
elastography:
a
meta-analysis
of
diagnostic
accuracy
in
evaluation
of
portal
hypertension
in
chronic
liver
disease.
Liver
Int,
2013.
33(1):
p.
62-71.
[5]
Smith,
J.O.
and
R.K.
Sterling,
Systematic
review:
Non-invasive
methods
of
fibrosis
analysis
in
chronic
hepatitis
C.
Alimentary
Pharmacology
and
Therapeutics,
2009.
30(6):
p.
557-76.
[6]
Stebbing,
J.,
et
al.,
A
Meta-analysis
of
Transient
Elastography
for
the
Detection
of
Hepatic
Fibrosis.
Journal
of
Clinical
Gastroenterology,
2010.
44(3):
p.
214-9.
[7]
Talwalkar,
J.A.,
et
al.,
Ultrasound-based
transient
elastography
for
the
detection
of
hepatic
fibrosis:
systematic
review
and
meta-analysis.
Clinical
Gastroenterology
and
Hepatology
2007.
5(10):
p.
1214-20.
[8]
Tsochatzis,
E.A.,
et
al.,
Elastography
for
the
diagnosis
of
severity
of
fibrosis
in
chronic
liver
disease:
A
meta-analysis
of
diagnostic
accuracy.
Journal
of
Hepatology,
2011.
54(4):
p.
650-9.
[9]
Lee,
C.K.,
et
al,
Serum
Biomarkers
and
Transient
Elastography
as
Predictors
of
Advanced
Liver
Fibrosis
in
a
United States
Cohort:
The
Boston
Children's
Hospital
Experience.
The
Journal
of
pediatrics,
2013.
163(4):
p.
1058-64.
[10]
Arena,
U.,
et
al.,
Acute
viral
hepatitis
increases
liver
stiffness
values
measured
by
transient
elastography.
Hepatology,
2008.
47(2):
p.
380-4.
[11]
Coco,
B.,
et
al.,
Transient
elastography:
a
new
surrogate
marker
of
liver
fibrosis
influenced
by
major
changes
of
transaminases.
Journal
of
Viral
Hepatitis,
2007.
14(5):
p.
360-9.
[12]
Mueller,
S.,
et
al.,
Increased
liver
stiffness
in
alcoholic
liver
disease:
differentiating
fibrosis
from
steatohepatitis.
World
Journal
of
Gastroenterology,
2010.
16(8):
p.
966-72.
[13]
Sagir,
A.,
et
al.,
Transient
elastography
is
unreliable
for
detection
of
cirrhosis
in
patients
with
acute
liver
damage.
Hepatology,
2008.
47(2):
p.
592-5.
[14]
Carrión,
J.A.,
et
al.,
Transient
elastography
for
diagnosis
of
advanced
fibrosis
and
portal
hypertension
in
patients
with
hepatitis
C
recurrence
after
liver
transplantation.
Liver
Transplantation,
2006.
12(12):
p.
1791-8.
[15]
Millonig,
G.,
et
al.,
Liver
stiffness
is
directly
influenced
by
central
venous
pressure.
Journal
of
Hepatology,
2010.
52(2):
p.
206-10.
[16]
Vizzutti,
F.,
et
al.,
Liver
stiffness
measurement
predicts
severe
portal
hypertension
in
patients
with
HCV-related
cirrhosis.
Hepatology,
2007.
45(5):
p.
1290-7.
[17]
Millonig,
G.,
et
al.,
Extrahepatic
cholestasis
increases
liver
stiffness
(FibroScan)
irrespective
of
fibrosis.
Hepatology,
2008.
28(5).
[18]
Lebray,
P.,
et
al.,
Liver
stiffness
is
an
unreliable
marker
of
liver
fibrosis
in
patients
with
cardiac
insufficiency.
Hepatology,
2008.
48(6):
p.
2089.
[19]
Mederacke,
I.,
et
al.,
Food
intake
increases
liver
stiffness
in
patients
with
chronic
or
resolved
hepatitis
C
virus
infection.
Liver
International,
2009.
29(10):
p.
1500-6.
[20]
Janssens,
E.,
et
al.,
Hepatic
amyloidosis
increases
liver
stiffness
measured
by
transient
elastography.
Acta
Gastroenterologica
Belgica,
2010.
73(1):
p.
52-4.
E300M017.4
03/2017
-
ECHOSENSTM
AND
FIBROSCAN®
ARE
TRADEMARKS
©
COPYRIGHT
ECHOSENS
-
ALL
RIGHTS
RESERVED
15
EE
FIBROSCAN
502
TOUCH
USER
MANUAL
FibroScan
[21]
Lanzi,
A.,
et
al.,
Liver
AL
amyloidosis
as
a
possible
cause
of
high
liver
stiffness
values.
European
Journal
of
Gastroenterology
and
Hepatology,
2010.
22(7):
p.
895-7.
[22]
Loustaud-Ratti,
V.R.,
et
al,
Non-invasive
detection
of
hepatic
amyloidosis:
FibroScan,
a
new
tool.
Amyloid,
2011.
18(1):
p.
19-24.
[23]
Fraquelli,
M.,
et
al.,
Reproducibility
of
transient
elastography
in
the
evaluation
of
liver
fibrosis
in
patients
with
chronic
liver
disease.
Gut,
2007.
56(7):
p.
968-73.
[24]
Nobili,
V.,
et
al.,
Accuracy
and
reproducibility
of
transient
elastography
for
the
diagnosis
of
fibrosis
in
pediatric
nonalcoholic
steatohepatitis.
Hepatology,
2008.
48(2):
p.
442-8.
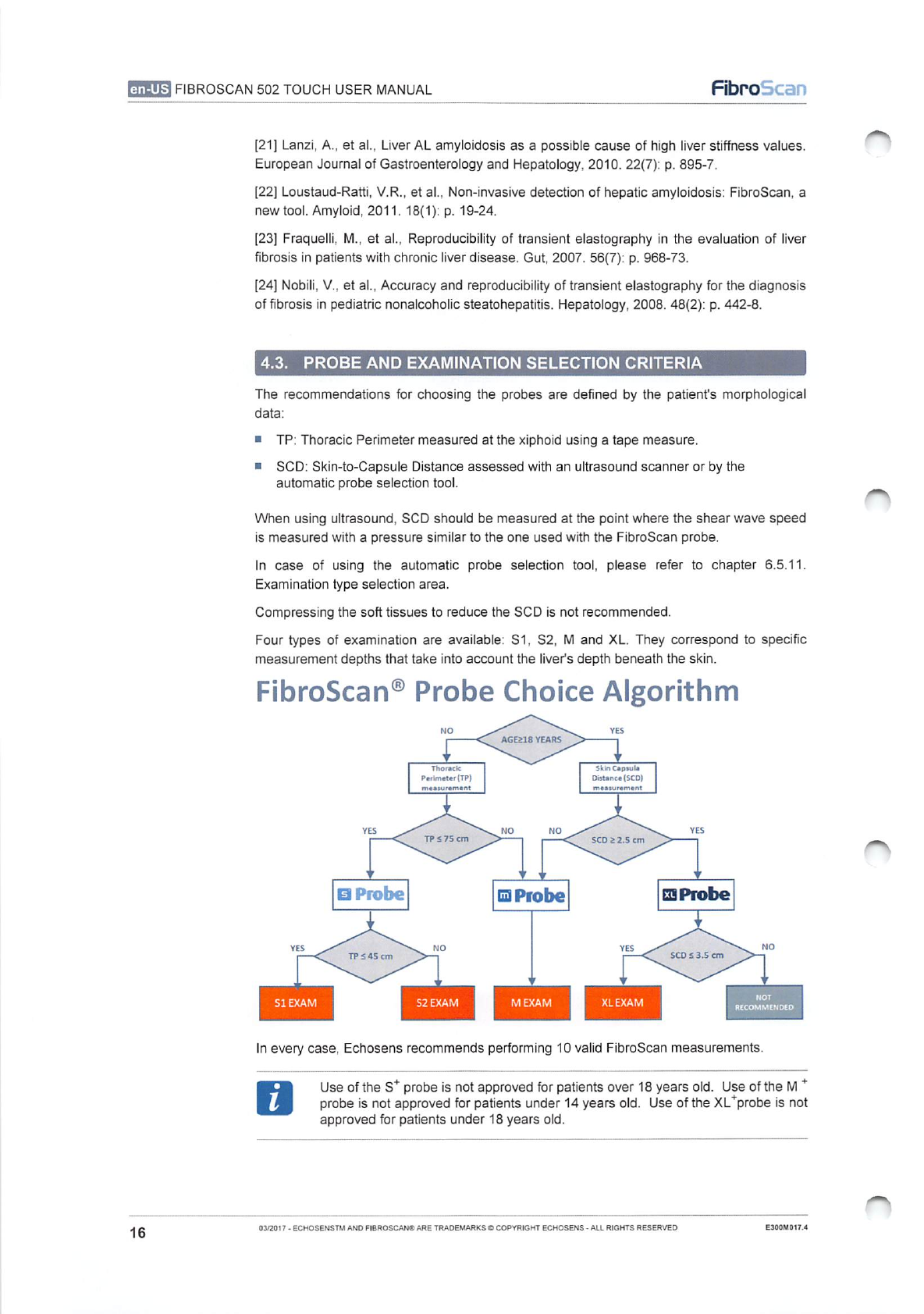
4.3.
PROBE
AND
EXAMINATION
SELECTION
CRITERIA
The
recommendations
for
choosing
the
probes
are
defined
by
the
patient's
morphological
data:
m
TP:
Thoracic
Perimeter
measured
at
the
xiphoid
using
a
tape
measure.
=
SCD:
Skin-to-Capsule
Distance
assessed
with
an
ultrasound
scanner
or
by
the
automatic
probe
selection
tool.
When
using
ultrasound,
SCD
should
be
measured
at
the
point
where
the
shear
wave
speed
is
measured
with
a
pressure
similar
to
the
one
used
with
the
FibroScan
probe.
In
case
of
using
the
automatic
probe
selection
tool,
please
refer
to
chapter
6.5.11.
Examination
type
selection
area.
Compressing
the
soft
tissues
to
reduce
the
SCD
is
not
recommended.
Four
types
of
examination
are
available:
S1,
S2,
M
and
XL.
They
correspond
to
specific
measurement
depths
that
take
into
account
the
liver's
depth
beneath
the
skin.
FibroScan®
Probe
Choice
Algorithm
No
YES
AGEz18
YEARS
Thoracic
Skin
Capsula
Perimeter
(TP)
Distance
(SCD)
measurement
measurement
[Cu
RECOMMENDED
S1EXAM
XLEXAM
In
every
case,
Echosens
recommends
performing
10
valid
FibroScan
measurements.
Use
of
the
S*
probe
is
not
approved
for
patients
over
18
years
old.
Use
of
the
M
+
probe
is
not
approved
for
patients
under
14
years
old.
Use
of
the
XL*probe
is
not
approved
for
patients
under
18
years
old.
16
03/2017
-
ECHOSENSTM
AND
FIBROSCAN®
ARE
TRADEMARKS
©
COPYRIGHT
ECHOSENS
-
ALL
RIGHTS
RESERVED
E300M017.4

FibroScan
FIBROSCAN
502
TOUCH
USER
MANUAL
ERETE]
4.4.
PRECAUTIONS
FOR
USE
The
following
instructions
must
be
followed
in
order
to
ensure
patient
safety.
The
FibroScan
should
not
be
used
in
the
following
situations:
On
any
organ
other than
the
liver.
The
eyes
and
mucosa
must
absolutely
be
avoided.
On
patients
with
active
implants
such
as
pacemakers,
defibrillators,
pumps,
etc.
On
wounds.
On
pregnant
women.
Moreover,
the
presence
of
ascites
between
the
probe
and
the
liver
may
prevent
from
obtaining
measurements
with
the
device.
The
clinical
personnel
must
follow
normal
safety
procedures.
The
FibroScan
examination
should
be
performed
carefully
using
the
principle
of
ALARA
(As
Low
As
Reasonably
Achievable).
4.5.
USER
TRAINING
Only
persons
having
received
training
in
the
use
of
the
FibroScan
and
in
possession
of
a
user
certificate
are
authorized
to
conduct
an
examination
using
the
FibroScan.
Training
is
essential
for
correct
equipment
use
and
in
order
to
obtain
reliable
and
reproducible
measurements.
This
manual
is
not
intended
to
provide
user
training.
4.6.
ELECTRICAL
SAFETY
The
FibroScan
is
manufactured
and
tested
in
accordance
with
IEC
electromagnetic
compatibility
(EMC)
and
electrical
safety
standards.
It
leaves
the
plant
in
full
compliance
with
safety
and
performance
requirements.
In
order
to
maintain
this
compliance
and
to
guarantee
the
safe
use
of
this
medical
device,
the
user
must
comply
with
the
indications
and
symbols
contained
in
this
manual.
A
Refer
to
the
warnings
in
Chapter
2
concerning
electrical
safety.
Prior
to
installation,
ensure
that the
operating
and
mains
voltage
values
match.
The
electrical
power
cable
provided
must
be
connected
to
the
FibroScan
mains
connector
and
to
a
grounded
socket.
Correct
grounding
can
only
be
guaranteed
if
the
FibroScan
is
connected
to
a
socket
compliant
with
safety
standards.
Safe
use
is
no
longer
guaranteed
in
the
following
main,
non-exhaustive
cases:
the
device
is
visibly
damaged,
the
device
is
inoperative,
after
prolonged
storage
in
unfavorable
conditions,
after
serious
damage
incurred
during
transport,
in
the
presence
of
flammable
or
anesthetic
gases.
This
may
cause
an
explosion.
Do
not
take
the
device
to
the
operating
room.
E300M017.4
03/2017
-
ECHOSENSTM
AND
FIBROSCAN®
ARE
TRADEMARKS
©
COPYRIGHT
ECHOSENS
-
ALL
RIGHTS
RESERVED
17

EE
FIBROSCAN
502
TOUCH
USER
MANUAL
FibroScan
If
the
FibroScan
can
no
longer
be
used
safely,
the
device
must
be
decommissioned.
Steps
must
be
taken
to
prevent
its
inadvertent
use.
The
medical
device
must
only
be
inspected
by
authorized
technicians.
4.7.
MAINTENANCE-RELATED
SAFETY
For
all
maintenance
operations,
the
physician
and
their
appointees
should
contact
Echosens,
who
will
send
an
authorized
technician.
For
correct
and
safe
use
and
any
maintenance
operations,
personnel
must
comply
with
normal
safety
procedures.
18
03/2017
-
ECHOSENSTM
AND
FIBROSCAN®
ARE
TRADEMARKS
©
COPYRIGHT
ECHOSENS
-
ALL
RIGHTS
RESERVED
E300M017.4

FibroScan
FIBROSCAN
502
TOUCH
USER
MANUAL
[GEIE]
5.
EXTERNAL
PRESENTATION
5.1.
HARDWARE
SUPPLIED
When
opening
the
package,
ensure
the
contents
match
the
following
list:
FibroScan
device
installed
Power
cables
according
to
country
Case(s)
fitted
with
probe(s)
Sealed
envelope
(Windows
EULA
license
and
this
user
manual)
Software
installation
CD
ROM
Set
of
four
fuses,
type
5x20
T2.0AH
250V
5.2.
ACCESSORIES
The
available
accessories
are:
=
S*
probe
u
MY
probe
=
XL? probe
Set
of
elements
that
can
be
connected
to
the
FibroScan
device:
A
FibroScan
Devices
not
included:
A:
DVI-I
screen.
B:
Overhead
projector.
C:
USB
storage
device.
D:
USB
printer.
Accessories:
E:
S*
probe.
F:
M*
probe.
G:
XL*
probe.
H:
Other accessories.
E300M017.4
03/2017
-
ECHOSENSTM
AND
FIBROSCAN®
ARE
TRADEMARKS
©
COPYRIGHT
ECHOSENS
-
ALL
RIGHTS
RESERVED
19

EE
FIBROSCAN
502
TOUCH
USER
MANUAL
FibroScan
5.3.
FRONT
VIEW
The
device
contains
the
electrical
power
supply,
dedicated
electronics
and
a
computer.
It
also
supports
a
monitor,
three
probe
holders,
and
a
gel
holder.
The
following
figure
presents
the
device's
various
user-accessible
parts.
_
CT
G
General
arrangement
of
the
FibroScan
device:
A:
Touch
screen.
B:
Caster
with
brake.
C:
Gel
holder.
D:
Standby
button
with
power
indicator.
E:
CD
ROM
/
DVD
ROM
drive.
F:
Computer
sockets.
G:
Probe
holder.
Standby
button
This
button
is
enabled
only
when
the
main
switch,
located
at
the
rear
of
the
device,
is
set
to
"I".
The
button
flashes
when
power
is
on.
Press
this
button
once
to
load
the
application;
the
built-in
indicator
light
turns
green.
After
a
few
seconds,
the
home
window
is
displayed.
Press
this
button
again
to
close
the
application;
the
built-in
indicator
light
and
monitor
are
both
turned
off.
This
is
the
usual
state
when
the
FibroScan
will
not be
in
use
for
a
short
time
(between
two
groups
of
patients,
for
example).
The
FibroScan
consumes
very
little
power
in
this
mode.
The
screen
and
the
software
This
is
a
17-
or
19-inch
color
LCD
touch
screen,
depending
on the
model
year.
To
protect
the
screen
from
any
risk
of
damage,
make
sure
not
to
hang
the
power
cable
on
the top
of
the
device.
The
FibroScan
runs
on
dedicated
software.
The
software
is
automatically
launched
when
the
FibroScan
is
switched
on.
It
is
used
to:
m
perform
examinations,
и
manage
examinations
in
archives.
20
03/2017
-
ECHOSENSTM
AND
FIBROSCAN®
ARE
TRADEMARKS
©
COPYRIGHT
ECHOSENS
-
ALL
RIGHTS
RESERVED
E300M017.4
Table of contents
Popular Medical Equipment manuals by other brands

OCULUS
OCULUS Pentacam AXL instruction manual

Olympus
Olympus EU-ME2 Quick reference guide

Cantel Medical
Cantel Medical MEDIVATORS ENDO STRATUS EGA-501 instruction manual

Basic American
Basic American ZENITH Series Instructions for use

HACH LANGE
HACH LANGE 8568200 manual

Albrecht
Albrecht CDS ALIGNMENT SET User instructions