EKF HemataStat II User manual

HemataStat II™
Centrifuge
Operator’s Manual
Separation Technology, Inc.
An EKF Diagnostics Company
570 Monroe Road, Suite 1008
Sanford, FL 32771
800-777-6668, 407-788-3677
www.separationtechnology.com

TABLE OF CONTENTS
SECTION 1 DESCRIPTION AND INTENDED USE ...........................2
SECTION 2 INSTALLATION..............................................................2
2.1 Unpacking
2.2 Power Supply
2.3 Optional Rechargeable Battery Pack
SECTION 3 SETUP PROCEDURE.....................................................5
3.1 Display Options
3.2 Setup Menu and Procedure
SECTION 4 OPERATING INSTRUCTIONS.......................................8
4.1 Start Up
4.2 Rotor Loading and Balancing
4.3 Tube Preparation
4.4 Tube Centrifugation
4.5 Reading a Capillary Tube
4.6 Hematocrit Determination Reminders
SECTION 5 QUALITY CONTROL AND ASSURANCE ...................15
5.1 Use of Hematology Reference Control
5.2 Calibration
5.3 Spin Time/RPM Tests
SECTION 6 MAINTENANCE............................................................16
6.1 Cleaning
6.2 Tube Holders
6.3 Inspections
6.4 Service
SECTION 7 TROUBLESHOOTING..................................................18
SECTION 8 ACCESSORIES, CONTROLS AND SUPPLIES...........19
SECTION 9 WARRANTY.................................................................20
SECTION 10 SPECIFICATIONS........................................................21

1
SECTION 1
DESCRIPTION AND INTENDED USE
The Separation Technology, Inc. (STI) HemataStat II™Microhematocrit System is a
small, lightweight centrifuge which provides a rapid and accurate microhematocrit
determination using capillary or venous blood samples. The HemataStat II is not
intended for use with materials other than blood.
The rotor will accommodate up to six standard 75mm capillary tubes. The device will
achieve specimen separation in only one minute.
For convenience and ease of use, the HemataStat II system also includes a built-in
automatictubereaderandaLCDthatdisplaysmessagestoguidetheoperatorthroughout
the testing procedure and then displays the test results.
An optional rechargeable battery pack is available to permit operation in remote locations
wherealternatingcurrentisnotavailable.TheHemataStatIIhasaCLIA'88WAIVEDstatus.
SECTION 2
INSTALLATION
2.1 UNPACKING
The HemataStat II shipping box contains:
•Centrifuge
•Package of ten disposable tube holders
•Power supply
•Operator’s manual
•Laminated quick reference procedure guide
•Laminated tube placement guide
•Instruction video
Read the operator’s manual thoroughly before operating this system.
VIEWINGTHEINSTRUCTIONVIDEOBEFOREPROCEEDINGWILLBEHELPFUL
FOR PROPER OPERATOR TRAINING.
Place the centrifuge on a convenient, level work surface along with the other items
contained in the box.

2
2.2 POWER SUPPLY
Use ONLY the power supply packaged with the HemataStat II. Verify that the
ON/OFF switch located on the top of the centrifuge is in the OFF " " position.
Alwaysplugthepowersupplyintotherearofthedevicefirstandthenplugthethree-
prongendintoanelectricaloutlet. PresstheON/OFFswitchtotheON"ʘ"position.
The lid will automatically open and the LCD will display the main menu.
2.3 OPTIONAL RECHARGEABLE BATTERY PACK
A. INSTALLATION
For complete portability, the HemataStat II will operate on a rechargeable
batterypack,whichcanbeorderedasanoption. Thebatterypackwillneedto
be installed in the device as described below and fullycharged before normal
operation.
1. Make certain the lid isclosed and locked andthe centrifuge isunplugged
from the electrical outlet. Lock the lid by firmly pressing down on the lid
tab.
2. Place the device upside down on a smooth,flat surfaceand on a cloth or
other protective material to prevent scratching the lid.
3. Locate the battery pack cover. Using a small head Phillips screwdriver,
remove the screws holding the battery pack cover in place. Retain the
screws. Remove the battery pack cover.
4. Connectthebatterypackplugintotheconnectorlocatedinsidethebattery
pack cavity. Put the connector inside the hole of the housing with the
connector release tab facing up. Position the battery pack inside the
compartment. Laythecablealongsidethebatterypacktoavoidcrimping.
5. Replace the battery pack cover and secure with the screws provided.
6. Plug in the HemataStat II and fully charge the battery pack.
B. CHARGING THE BATTERY PACK
ThebatterypackisalwayschargingwhenevertheHemataStatIIispluggedinto
an electrical outlet. Since the battery pack cannot be overcharged, the device
may remain plugged in continuously without harm. Charge the battery pack
overnight (or an equivalent period of time). The percentage of remaining
battery pack capacity may be determined at any time by performing the
following procedure.
3
1. Unplug the device from the electrical outlet.
2. PresstheON/OFFswitchtotheONposition. TheLCDshoulddisplaythe
main menu.
ENT
ENTER FUNCTION:
READ 1.1 (OR 0.5) SPIN
RUN
3. PresstheRUNandENTbuttonssimultaneouslyandholdthemdownuntil
the battery pack capacity is displayed.
When there is 20%capacity remaining, the main menu LCD will flash as
an indication that the battery packis beginning torun down.It will still be
possible to operate the device for a few spins but the operator should
recharge the battery pack soon. Once there is no longer sufficient
capacity to operate the instrument, it will fail to complete a cycle and the
message CHARGE BATTERY will be displayed.
4. Ifthedeviceis pluggedintoanelectricaloutletwiththeON/OFFswitchin
the ON position and the RUN and ENT buttons are pressed
simultaneously, the LCD will display either CHARGING when in the
process of charging or TRICKLECHARGING if fully charged.
REGARDLESS OF WHAT THE LCD MAY DISPLAY AT ANY GIVEN
TIME DURING THE CHARGING CYCLE, IT IS IMPORTANT TO
ALWAYSALLOWTHEBATTERYPACKTOCHARGEOVERNIGHTOR
AN EQUIVALENT PERIOD OF TIME TO ENSURE A FULL CHARGE.
AFULLYCHARGED BATTERYPACKWILLGENERALLYPROVIDE75
SPIN CYCLES IF THE CENTRIFUGE IS TURNED OFF BETWEEN
TESTS.
BATT. CAPACITY
XX %
4
SECTION 3
SETUP PROCEDURE
3.1 DISPLAY OPTIONS
Thissetupprocedurewillallowtheoperatortostoreseveralfunctionsinthedisplay
option memory until a change is required. These functions include the selection of:
•Tubesize-1.1mmor0.5mmInsideDiameter(ID).Thedevicemustbesetto
the tube size being used. (The ID ofcapillary tubesmay vary slightly -up to
0.1mm.)
•Display language - English, Spanish, French, German or Italian
•HCT value suffix - Choice of displaying %sign or only the value itself
•Decimal point - Choice of displaying hematocrit in whole numbers or with a
decimal point.
Performingthissetupprocedureeliminatestheneedtoenterthesesettingsintothe
device each time a test isperformed.
3.2 SETUP MENU AND PROCEDURE
A. With the power ON, the LCD will display the main menu:
ENT
ENTER FUNCTION:
READ 1.1 (OR 0.5) SPIN
RUN
B. SimultaneouslypressandreleasetheENTandRUNbuttons.TheLCD
will change to:
ENT
SETUP
TUBE I.D. NEXT
RUN
5
C. PressENTtoenterthetubesizemenu. PressENTagaintochoosethe
1.1mm size or press RUN for 0.5mm.
ENT
SELECT TUBE I.D.
1.1mm 0.5mm
RUN
When the tube size selection is made, the LCD will return to the main
menu.
D. SimultaneouslypressandreleasetheENTandRUNbuttons.TheLCD
will change to:
ENT
SETUP
TUBE I.D. NEXT
RUN
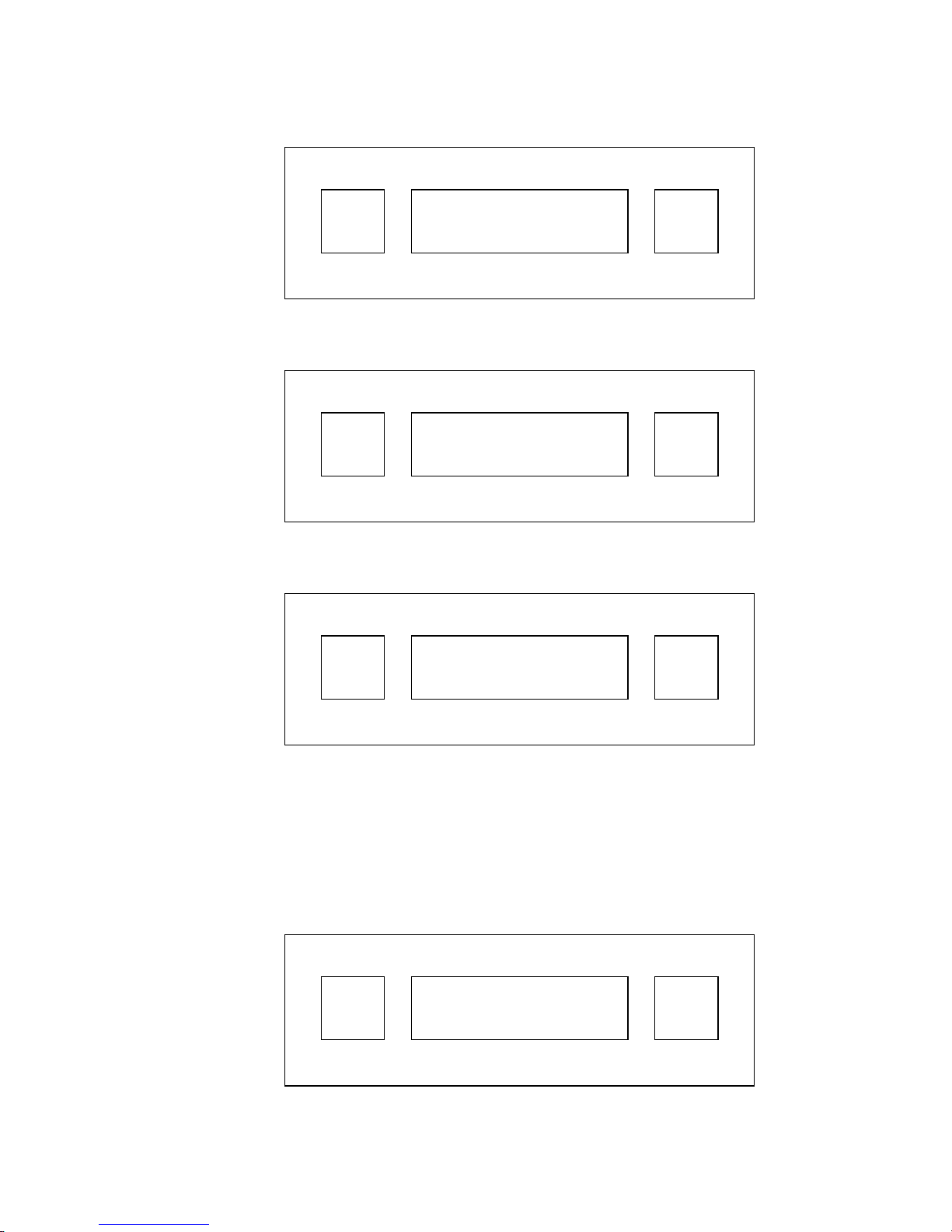
E. Press RUNto enter the language menu. The LCD will display:
ENT
SETUP
LANGUAGE NEXT
RUN
F. Press ENT to select language display, then press RUN repeatedly to
display each of the 5 language options. Press ENT to lock in the
desired language. The LCD will return to the main menu.
6
G. SimultaneouslypressandreleasetheENTandRUNbuttons. TheLCD
will change to:
ENT
SETUP
TUBE I.D. NEXT
RUN
H. Press RUNtwice to enter the HCT suffix menu. The LCD will display:
ENT
SETUP
% SIGN NEXT
RUN
I. Press ENT to make HCT suffix selection. The LCDwill display:
ENT
HCT % SIGN?
YES NO
RUN
J. Press ENT again to lock in the %suffix or press RUN to remove the%
suffix. When the option is selected, the LCD will return to the main
menu.
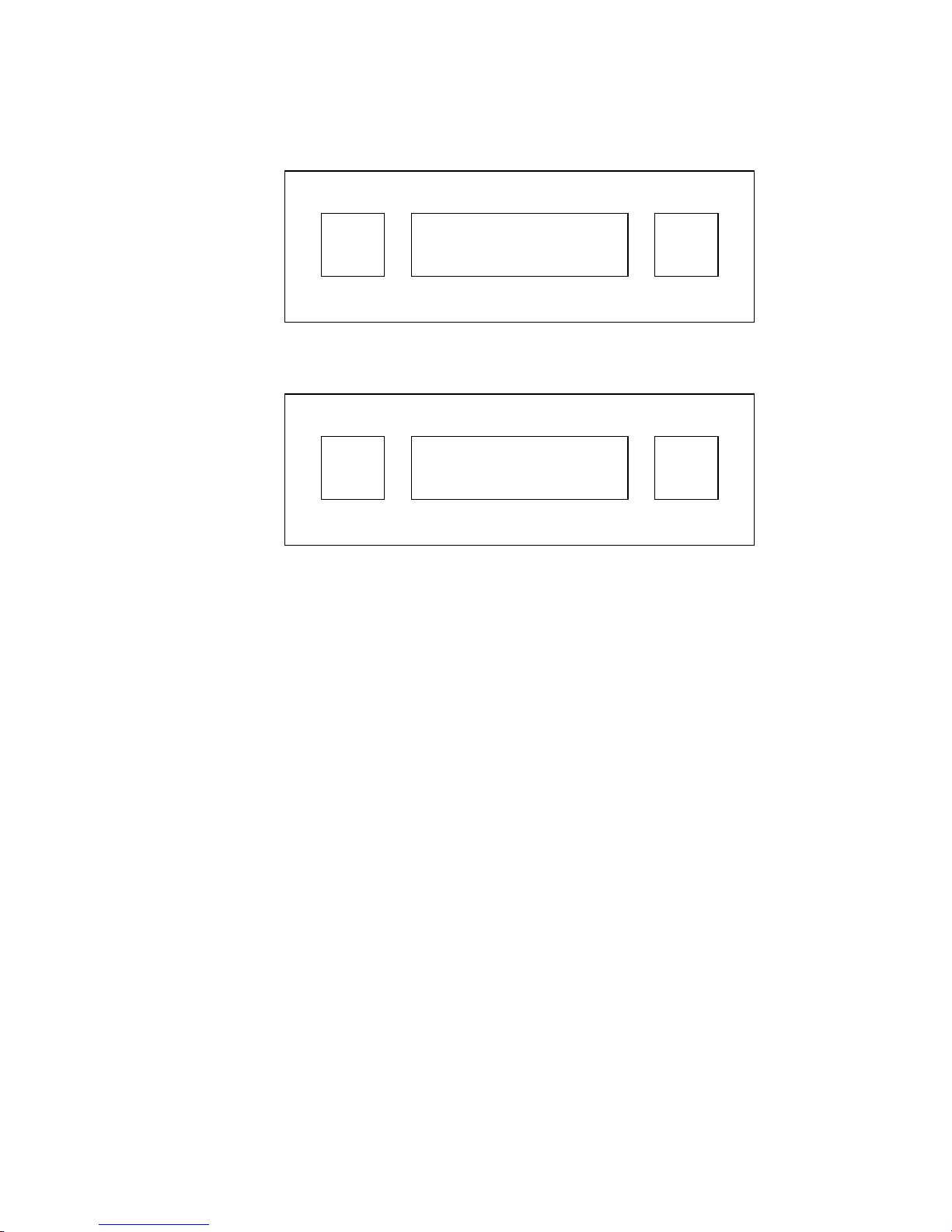
K. SimultaneouslypressandreleasetheENTandRUNbuttons. TheLCD
will change to:
ENT
SETUP
TUBE I.D. NEXT
RUN
7
L. Press RUNthree times to enter the decimal point menu. The LCDwill
display:
ENT
SET UP
DECIMAL NEXT
RUN
M. Press ENT to make decimal point selection. The LCD will display:
ENT
DECIMAL POINT?
YES NO
RUN
N. Press ENT again to lock in the decimal point or press RUN toremove
the decimal point. When the option is selected, the LCD will return to
the main menu.
SECTION 4
OPERATING INSTRUCTIONS
4.1 START UP
PresstheON/OFFswitchtotheONposition. Thelidwillautomaticallyopenandthe
LCD willdisplay the main menu.
THE INSIDE DIAMETER (ID) OF THE TUBES BEING USED MUST MATCH THE
SIZE DISPLAYED ON THE LCD (EITHER 0.5 OR 1.1MM) (SEE SECTION 3.2).
4.2 ROTOR LOADING AND BALANCING
FOR SMOOTHOPERATIONANDEXTENDEDLIFEOF THECENTRIFUGE,THE
ROTOR MUST ALWAYS BE BALANCED BEFORE THE SPIN CYCLE IS
INITIATED.
Install2,4or6tubeholdersintherotor. Thereshouldalwaysbeanevennumberof
tubeholdersintherotorandtheyshouldbeoppositeeachothertobalancetherotor.

8
When an even number of capillary tubes (2, 4 or 6) are centrifuged, balancing is
accomplished by placing the tubes on opposite sides of the rotor from one another
so that the weight is distributed equally. If an odd number of tubes (1,3 or 5) are
centrifuged, use an empty capillary tube to balance the rotor.
4.3 TUBE PREPARATION
A. Perform a capillary finger stick.
1. The patient should be seated comfortably. The patient’s hand should
be below the heart. It should be warm and relaxed with the fingers
straight but not tense. Warm the finger by moving the blood from the
base to the tip several times.
2. The puncture site should be on the fingertip of the middle finger or ring
finger. Ideally, there should not be a ring on the finger. The outer and
upper region of the fingertip, halfway between the center of the finger
pad and the edge of the fingernail, is the site of choice. Using the side
of the fingertip rather than the center of the fingertip hurts less and
provides more blood flow.
3. Clean the puncture site with an alcohol swab. Allow the area to dry
completely or wipe it off with a lint free wipe (such asgauze).
4. Using your thumb, lightlypress the finger from above the knuckle to the
tip to stimulate blood flow toward the puncture site.
5. While applying light pressure toward the puncture site, use the lancet.
B. Wipe off the first drop of blood. Fill a capillary tube ½ to ¾ full with blood. Let
the specimen flow down the tube until it is near the dry end. Then placeyour
finger over the top of the tube to stop the flow.
C. Insertthedryendverticallyintothesealant,pushingittothebottomofthetray.
Twist the tube when removing it from the sealant to prevent the sealing plug
from being extracted.
D. Repeat Step B. Gently tap the sealed end of the tube ona flatsurfaceto help
insure proper sealant contact in the tube.
E. Wipe off the prepared capillary tube.
F. Place the capillary tube carefully in the centrifuge tube holder with the sealant
end down. Do not force the tube; let it slide into the tube holder. All tube
positions are numbered on the rotor and can be used to record the position of
each patient specimen.
WHEN USING CAPILLARY TUBES WITH PAINTED FILLER BANDS DO NOT
FILL THE TUBES MORE THAN ¾ FULL. SEAL THE END OF THE TUBE
WITHOUT THE BAND.
WHEN DRAWING A SAMPLE FROM A VENOUS BLOOD COLLECTION TUBE

9
ENSURE THAT THE SAMPLE IS WELL MIXED.
4.4 TUBE CENTRIFUGATION
With the tube holders and hematocrit tubes in place, lock the lid by firmly pressing
downonthelidtab. StarttheruncyclebypressingtheRUNbutton. Thecentrifuge
will not operate unless the lid is closed and properly locked. If the RUN button is
pressed without locking the lid, the message LOCK LIDwill appear on the LCD.
Do not lean on the instrument.
Duringtheperiodoftimewhenthemotorisaccelerating,atestnumberisdisplayed
on the LCD. This number is a count of the completed spin cycles. Within seconds
thecentrifugewillreachtheproperoperatingspeedandtherpmwillbedisplayedon
the LCD during the cycle. The LCD will display a countdown of time for the
remainder of the run cycle, and thedevice willstop automaticallyafter 60 seconds.
When the run cycle is completed, an automatic braking system will engage to
completely stop the rotor. An audible tone will indicate that the spin cycle is
complete and the automatic lid lock will disengage to allow the lid to be opened.
4.5 READING A CAPILLARY TUBE
A. Look at the LCD to insure that the proper tube size (1.1 or 0.5mm)
appears in the LCD between the READ and SPIN messages.
ENT
ENTER FUNCTION:
READ 1.1 (OR 0.5) SPIN
RUN
B. Move the slider to the far left side of the reader tray.
ONLY ONE CAPILLARY TUBE AT A TIME SHOULD BE REMOVED
FROM THE ROTOR FOR READING. ADDITIONAL TUBESMAY BE
LEFTINTHEROTORFORUP TOFIVE(5)MINUTESWITHOUTANY
ADVERSE EFFECTS. ONCEATUBEHASBEENREMOVEDFROM
THE ROTOR, IT SHOULD BE READ WITHIN ONE MINUTE.
C. Remove a capillary tube from the rotor and place it in the groove
located in front of the LCD.Make sure the sealant endof the tubeis to
thefarleft,againsttheendofthegroove. Rotatethetubeinthegroove

10
so that the full diagonal interface of the Red Blood Cells
(RBCs)/PLASMA can easily be seen as shown below:
Note that the diagonal can be in this position or in this position,
whichever way provides the clearest view of the interface.
Onceithasbeenproperlypositioned,makesureyoudonotmovethe
tube during the reading process.
D. Press the ENT button. The LCD will change to:
ENT
ENTER INTERFACE:
SEALANT/RBCs
RUN
Move the slider along the capillary tube to the interface of the tube
sealant and red blood cells. Look through the transparent slider and
positiontheverticalblacklineontheinterfaceasshowninthefollowing
diagram.

11
E. Press the ENT button. The LCD will change to:
ENT
ENTER INTERFACE:
RBCs/PLASMA
RUN
F. Move the slider to the RBCs/PLASMA interface. Look through the
transparent slider and position the vertical black line on the middle of
the diagonal interface as shown in the following diagram.
In some instances, a line of red blood cells extending from the
RBCs/PLASMA interface through the PLASMA/AIR interface can be
observed. These fine lines of red blood cells are residuals from the
migration and they have not been found to affect the results.
G. Press the ENT button. The LCD will display:
ENT
ENTER INTERFACE:
PLASMA/AIR
RUN
H. Move the slider to the PLASMA/AIRinterface. Place thevertical black
line of the slider over the interface at the end of the plasma curve as
shown in the following diagram.

12
I. Press the ENT button. The LCD will displaythe hematocrit result.
ENT
HCT = XX%
NEXT TUBE MENU
RUN
J. Press ENT to read another tube. Press RUN to return to the main
menu.
4.6 HEMATOCRIT DETERMINATION REMINDERS
TO ENSURE CORRECT RESULTS, BE SURE TO:
•Use only the size tube displayed on the LCD.
•Use only heparinized capillary tubes.
•Spin sample one time (60 seconds) ONLY.
•Read the tube within one minute after removing it from the rotor.
•Align vertical black line of the slider at the middle of the RBCs/PLASMA full
diagonal interface.
•Tubes spun in HemataStat must be read on the HemataStat.
•TubesspuninanyotherbrandofcentrifugecannotbereadontheHemataStat.
•Inspectandreplacedisposableplastictubeholdersmonthlyormorefrequently
as needed.

13
SECTION 5
QUALITY CONTROL AND ASSURANCE
QUALITYCONTROL ANDASSURANCETESTSARELEFTTOTHESOLEDISCRETION
OF THELABORATORYDIRECTORWHERETHEHEMATASTATIIMICROHEMATOCRIT
SYSTEM IS IN USE.
5.1 USE OF HEMATOLOGY REFERENCE CONTROL
To insure proper daily performance of the HemataStat II, STI provides a
hematologyreferencecontrolcalledHemataChek™. Theproducthasanassayvalue
for the hematocrit test and is packaged in a variety of combinations of LOW,
NORMAL and HIGH (See Section 8).
The HemataStat II’s accuracy and the user’s technique can be confirmed by using
HemataChek hematology reference control.
HemataChekdoes not require refrigeration and features a 2 year expiration from
date of manufacture and a 31 day open vial stability. It provides hematocrit assay
values for all HemataStat centrifuges as well as for other microhematocrit
centrifuges.
WHEN USING HEMATACHEK:
Step 1 Ensure bottle cap is tightly closed.
Step 2 Vigorously tap the bottle against the palm of yourfree hand. Assoon as
the plastic mixing bead can be heard, continue to tap the bottle for one
minute.
Step 3 After mixingone minute, look throughthe bottom ofthe bottle. If a clump
of unmixed control material can still be seen, repeat Steps 1 and 2.
Step 4 Use this mixing technique each time before filling capillary tubes.
Step 5 Aftereachuse,cleanthethreadsofthevialandthecapwithanabsorbent
material.
Step 6 Always replace the cap after use.
5.2 CALIBRATION
Microprocessor technology is used to monitor the speed and spin cycle of the
HemataStat II. A maximum packing time test is not applicable. If an abnormal
operatingconditionisencountered,aprogrammederrormessagewillautomatically
appearinthedisplay. TheaccuracyoftheHemataStatIIhematocrittubereadercan
beverifiedbyusingHemataChekhematologyreferencecontrol,availablefromSTI.

14
5.3 SPIN TIME/RPM TESTS
A. SPIN TIME
Spintimeisfactorysetat60+/-3seconds. Acountdownofthetimeremaining
isdisplayedontheLCD. Spintimemaybeverifiedbyusingastopwatch. The
motortakeslessthan10secondstoacceleratetoproperrpm. Onceachieved,
thespintimewillbedisplayedontheLCD. StarttimingthespinwhenWAIT60
SEC is displayed on the LCD. Stop timing the spin when the motor shuts off.
B. RPM
The HemataStat II is designed to operate between 5,670 and 6,930 rpm. An
internalmicroprocessorcontinuouslymonitorstherpmduringoperation.Ifthe
rpm should drop below the specified range, the motor will shut off, and the
messageLOWRPMwilldisplayontheLCD.ToverifytheLOWRPMmessage,
press the ENT or RUN button to return to the main menu. Press the RUN
button to restart the spin cycle. If the LOW RPM message is displayedagain,
refer to Section 7.
The rpm reading on the LCD should be within 2%of a tachometer reading.
SECTION 6
MAINTENANCE
6.1 CLEANING
Aswithallelectricaldevices,makesurethecentrifugeisunpluggedbeforecleaning.
Always wear protective clothing when using anycleaning materials.
NEVER USE BLEACH, ABRASIVES OR CORROSIVE SOLVENTS.
DO NOT SPRAY OR ALLOW ANY LIQUID TO GET INSIDE THE CENTRIFUGE.
LIQUIDWILLHARMTHEELECTRONICS.SUBSEQUENTPROBLEMSWILLNOT
BE COVERED UNDER WARRANTY.
Use a disinfectant towelette or a cloth slightly dampened with any non-corrosive
disinfectantsolutiontocleanthelidandotherpartsofthecentrifugehousing. Dryall
surfaces with a soft tissue or cloth after cleaning.
The rotor should be removed and cleaned at least monthly or as required by the
laboratory protocol. Remove the rotor from the motor shaftbyfirst unscrewing the
rotorknob. Gentlylifttherotorverticallyoffofthemotorshaft. Makesuretherotoris
thoroughly dry before reinstalling. Liquid left on the rotor willcause damageto the
device. Re-install the rotor making certain that the rotor knob is tight.

15
6.2 TUBE HOLDERS
Cleaning the tube holders isnot recommended. Should a capillary tube break ora
sealant blowout occur, simply discard the affected tube and tube holder in
accordance with proper laboratory procedures and replacewith a newtube holder.
Inspect tube holders regularly and replace them when they become dirty and/or
contaminated. Replacement tube holders are available (See Section 8).
6.3 INSPECTIONS
Periodically inspect the lid, lid gasket and rotor to ensure there are no cracks or
damage.
6.4 SERVICE
To obtain service, contact Customer Service at 800-777-6668, 407-788-8791, or
by email at [email protected].
Allinstrumentsoraccessoriesmustbecleanedpriortoshipmenttothemanufacturer
forservice. ThisdecontaminationisrequiredbyFederallawandEPARegulations.
Employees of STI cannot perform thisdecontamination.
When transporting the HemataStat II, removal of the rotor or placement of packing
materialaroundtherotorwillhelppreventdamagetothemotorshaftintheeventthe
unit is dropped.

16
SECTION 7
TROUBLESHOOTING
SYMPTOM PROBLEM SOLUTION
No display
Power supply not firmly plugged into
electrical outlet or in back of device
Power supply not functioning
ON/OFF switch not ON
Faulty electrical outlet
Check both plugs
Replace power supply.
Turn ON/OFF switch ON
Try a different electrical outlet
Rotor will not spin Lid not locked Press firmly down on the lid tab.
Unit Noisy
Rotor knob is not tight
Rotor not balanced
Tighten rotor knob
Balance the rotor
Lid will not open
Device is in the spin cycle
Lid lock is engaged
Allow spin cycle to end (Section 4.4)
Turn ON/OFF switch off, wait 5
seconds and turn it back on.
Use the key tool on the underside of
the unit. Insert the “L” shaped end of
the tool into the ”L” shaped key opening
on the left side of the unit. Push gently
until the lid opens.
LOW RPM
message Low rpm
Insure that the rotor moves freely
Check rotor balance
RUN ABORTED
message
Power failure
Power supply disconnected
Restore power
Reconnect plug
ENTRY ERROR
message
If the slider is moved out of sequence
or is moved in the wrong direction.
Move the slider to the far left and start
the reading process over. (Section 4.5)
LCD flashing
Low battery pack charge
(less than 20% capacity)
Power supply not firmly plugged into
electrical outlet or in back of device
Recharge or replace battery pack
Check both plugs
Try a different electrical outlet
Battery pack will
not charge
Power supply not properly connected
Battery pack not properly connected
Faulty battery pack
Section 2.2 - Power Supply
Section 2.3 - Optional Rechargeable
Battery Pack
Replace battery pack
Battery pack
does not hold
adequate charge Voltage is inadequate to charge
HemataStat II Plug in fewer power supplies in
electrical outlet (same circuit)

17
SECTION 8
ACCESSORIES, CONTROLS AND SUPPLIES
CONTROLS HemataChek™Hematology Control is specific for monitoring the Hematocrit
determination and is stable for 2 years from date of manufacture at room temperature. Open
vial stability is 31 days. Each box contains six 2.5ml vials.
300-101 HemataChek - Normal Control Level, 6 Vials, 2.5ml ea.
300-102 HemataChek - Low, Normal & High Control Levels, 2 Vials/Level, 2.5ml ea.
300-103 HemataChek - Low & Normal Control Levels, 3 Vials/Level, 2.5mlea.
300-104 HemataChek - Normal & High Control Levels, 3 Vials/Level, 2.5ml ea.
300-105 HemataChek - Low Control Level, 6 Vials, 2.5ml ea.
300-107 HemataChek - High Control Level, 6 Vials, 2.5ml ea.
SUPPLIES
230-100 Tube Holders - 50/Pack
260-100 HemataSeal™Tube Sealant
260-105 Unistik®2 Lancets, Normal, 100/Box
260-109 Unistik®2 Lancets, Dual, 200/Box
260-110 Unistik®2 Lancets, Extra, 200/Box
260-111 Unistik®2 Lancets, Extra, 1,000/Box
270-106 ClearCrit™Plastic Microhematocrit Capillary Tubes, Heparinized,
75mm length, 1.1mm ID, Box of 5 Vials; 200 Tubes/Vial
270-107 ClearCrit™PlasticMicrohematocrit Capillary Tubes, Heparinized,
75mm length, 0.5mm ID, Box of 5 Vials; 200 Tubes/Vial
270-108 ClearCrit™Mylar®Coated Glass Microhematocrit Capillary Tubes,
Heparinized, 75mm length, 1.1mm ID, Box of 5 Vials; 200 Tubes/Vial
270-109 ClearCrit™Self Sealing, Mylar®Coated Glass Microhematocrit Capillary Tubes,
Heparinized, 75mm length, 1.1mm ID, Box of 20 Vials; 100 Tubes/Vial
75mm length, 0.5mm ID, Box of 5 Vials; 200 Tubes/Vial
ACCESSORIES
280-104 Rechargeable Battery Pack
290-111 Power Supply Assembly - 100-240VAC / 47-63Hz 0.7A
320-100 Carrying Case - Holds 2 Centrifuges
320-101 Training Video
630-124 Key Tool
910-100 Operator Manual
Mylar® is a registered trademark of E.I. du Pont de Nemours and Company

18
SECTION 9
WARRANTY
SEPARATION TECHNOLOGY, INC. (STI) warrants each new HemataStat II™ (The
Product)againstdefectsinmaterialsorworkmanshipforaperiodoftwoyearsfromthedate
of purchase and agrees to repair or replace any defective Product without charge. This
warranty does not cover damage resulting from accident,misuse, lackofreasonablecare,
impropercleaning,impropermaintenanceorimproperpackagingforreturnshipmenttoSTI.
This warranty shall be void if the Product is repaired by anyone other than STI or an
authorized service agent. This warranty does not extend to anyone other than the original
purchaser nor to accessories manufactured by other vendors.
Excludedfromthistwoyearproductwarrantyistheoptionalrechargeablebatterypack.This
itemiswarrantedbySTIagainstdefectsinmaterialor workmanshipforaperiodof90days
from date of purchase.
Except as provided herein, STI makes no warranties of any kind, either expressed or
implied,andspecificallyexcludinganywarrantyofmerchantabilityorwarrantyoffitnessfora
particular purpose.
STIwillnotbeliablefor any special,consequentialor incidentaldamagesarisingoutofthe
useorinabilitytousetheProductand/ortheoptionalrechargeablebatterypack. Innoevent
shallSTI'sliabilityhereunderexceedthepurchasepriceoftheProduct. Thiswarrantyshall
be void and of no force and effect with respect to any Product and/or the rechargeable
batterypackwhichisdamagedasaresultofa)neglect,alteration,electriccurrentfluctuation
or accident, b) improper use, including failure to follow proper operation andmaintenance,
and to provide proper environmental conditions prescribed in STI's Product instruction
manuals, c) repair by other than STI or authorized service agents appointed by STI and
actinginaccordancewithSTI'sserviceannouncementsord)useofsuppliesorpartswhich
do not meet STI specifications.
To obtain warranty service, contact Customer Service at 800-777-6668, 407-788-8791,
HemataStat II, HemataChek, HemataSeal and ClearCrit are trademarks of Separation Technology, Inc.
Table of contents
Popular Laboratory Equipment manuals by other brands
Hanna Instruments
Hanna Instruments HI931 user manual
Miele
Miele A 306/1 operating instructions
Brooks
Brooks CoolCell SV10 Instructions for use
Merck
Merck APC SmartTouch Operation manual

Diesse
Diesse cube 30 touch quick start guide

Micromeritics
Micromeritics Saturn II 5205 DigiSizer Installation Instructions and Checklist