PRODUCT DESCRIPTION
NP-DO M-39_12-2021 10 12/2021
PRODUCT DESCRIPTION
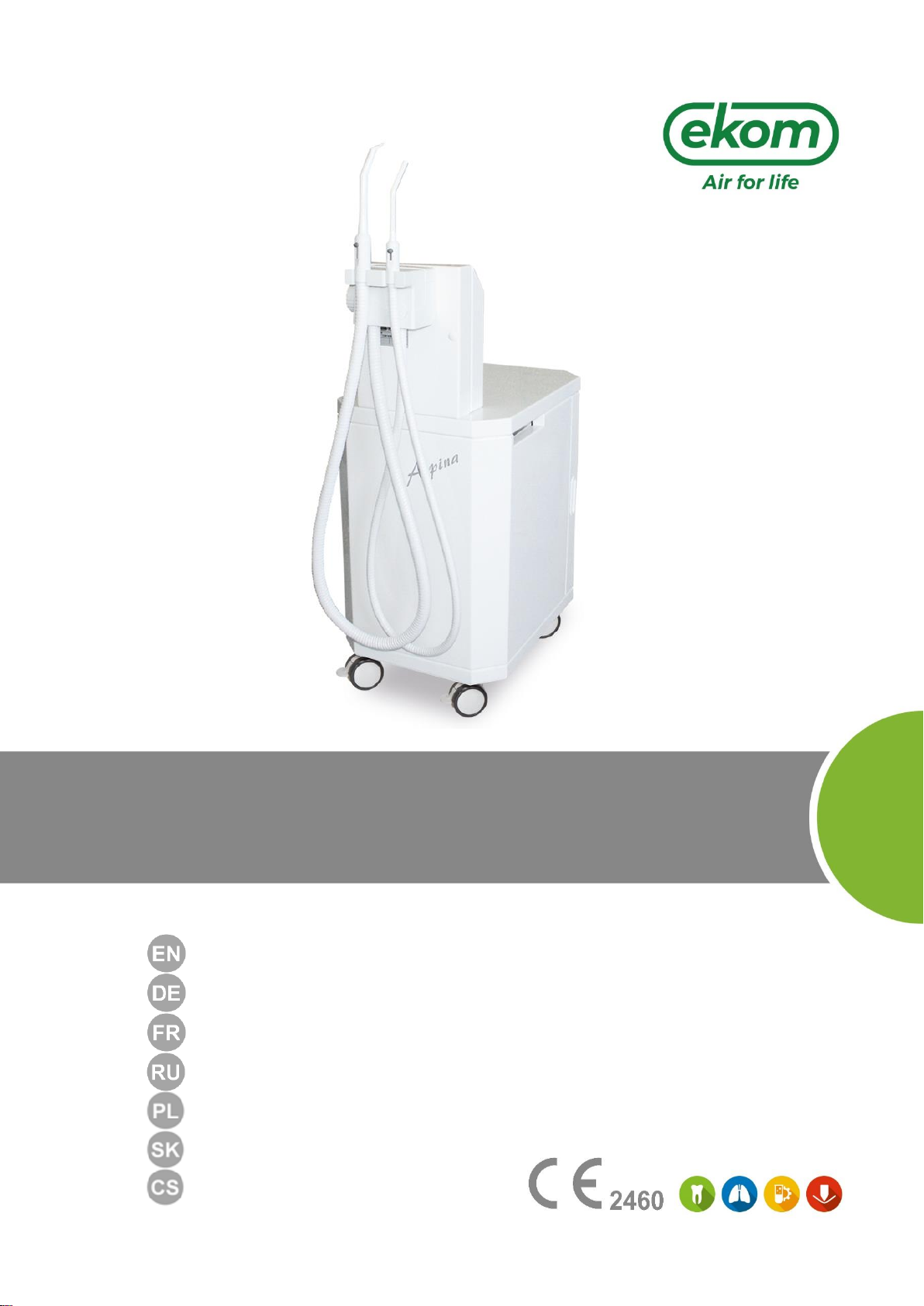
Fig. 1: The mobile dental suction equipment ASPINA DO M is built-up on a movable base in a noise
damping cabinet. Inside the cabinet there is situated a fan cooled suction pump (9) with the electric
distribution and the waste entrapping separation vessel (11). In the lower part –under the box –
there is situated a silencer with an output filter (14) and a pre-filter (15) ensuring the air filtration
from the suction pump. In the upper –narrowed part of the suction equipment - there is situated a
holder for suction tubings (2) equipped with cannulas (1), separation automatics and therminal box
with fuses. Onthe lateral part there is situated a main switch (5), over which are placedthe indicators
for the network (3) and for the state of filling of the separation vessel (4).
PRODUCT FUNCTION
7.1. Function Description
Fig. 1: After switching-on the main switch (5) into the position „I“ the network indicator (3) goes on.
Taking the suction tubing (6) off the holder (2) will actuate the suction pump (9) and the vacuum
occurs at the suction mouthpiece (1). After repeated putting the suction tubing into the holder, the
suction pump turns off. When the separation vessel (11) is filled with waste products, the suction
pump turns off and the indicator for the separation vessel (4) filling goes on. Then it is necessary to
return the suction tubing back into the holder and to empty the separation vessel. During a longer
work, mainly with the desalivating mouthpiece, the box temperature may increase; the cooling fan
is then automatically actuated. The fan turns off automatically, when the box temperature drops.
7.2. Detailed Description of the Suction Part Function
Fig. 1: The vacuum air flows together with sucked waste products from the oral cavity through the
tubing system from the suction mouthpiece (1) at first through the inlet sieve (7), wherein the solid
impurities are entrapped. The vacuum air, together with the sucked waste products, free of solid
impurities greater than 2 mm, then flows into the separation vessel (11) wherein it is separated from
the vacuum air and entrapped into the separation vessel.
Suction air flows through the filter (18) and into the suction unit (9), from which it is pushed through
a noise muffler. Therein the output air passes through the output pre-filter (15) and the bacteriologic
output filter (14). After passing the filters, the airfree of impurities is blown off into afree space under
the dental suction equipment.