Idmed Algiscan User manual

User Manual AlgiScan 1
USER MANUAL
AlgiScan
Version 3.2 - EN
Last update 2021/04/19
Ref: ALG-IFU_EN
USER MANUAL

User Manual AlgiScan 2

User Manual AlgiScan 3
CONTENTS
About this manual............................................................................................... 5
Indications of use................................................................................................. 5
Expected performance....................................................................................... 5
Clinical Benets..................................................................................................... 5
Important information about the use of AlgiScan ............................... 6
I Safety measures ................................................................................................ 6
I.1 Warnings..........................................................................................................................................7
I.2 Caution........................................................................................................................................... 8
I.3 Explanation of symbols.........................................................................................................10
II General description ........................................................................................12
Overview of the AlgiScan and its accessories............................................................... 12
Display screen .................................................................................................................................12
Touch screen.................................................................................................................................... 12
General use.......................................................................................................................................13
Fitting the positioning eyecup.................................................................................................13
Installation and positioning of the AlgiScan....................................................................13
Turning the device ON/OFF.......................................................................................................14
Patient Files / Measurements identication....................................................................14
Settings................................................................................................................................................14
III Using the AlgiScan.........................................................................................15
Create or select a patient’s le...............................................................................................15
Taking a measurement ..............................................................................................................16
Hold function ....................................................................................................................................16
Measurement protocols............................................................................................................. 17
Selecting the Measurement Protocol..................................................................................18
“Flash” mode .....................................................................................................................................18
“PRD” mode ........................................................................................................................................19
“Tetanus” mode...............................................................................................................................19
« PPI » Mode.......................................................................................................................................21
Connection and positioning of the stimulation electrodes ...................................22
Display of the results...................................................................................................................24
Results in the “Light ash” stimulation mode .................................................................24

User Manual AlgiScan 4
Results in the “Tetanus” mode................................................................................................26
Results in “PRD” mode .................................................................................................................27
Results in the “PPI” mode...........................................................................................................28
Review saved les / Trend charts........................................................................................29
Selecting a patient’s le.............................................................................................................29
IV Preventive maintenance, cleaning and disinfection ...................30
Preventive maintenance...........................................................................................................30
Battery and battery charging.................................................................................................30
Cleaning and Disinfection.........................................................................................................31
V Appendix 1..........................................................................................................32
Troubleshooting.............................................................................................................................32
VI Appendix 2........................................................................................................33
End of life equipment / Recycling........................................................................................33
Specication and warranty .....................................................................................................33
Environment.....................................................................................................................................33
Technical specications............................................................................................................34
VII Appendix 3 : accessories..........................................................................37

User Manual AlgiScan 5
About this manual
This operating manual contains all the information necessary to set up and use the
portable pupillometer AlgiScan manufactured by IDMED. It also describes the specic cleaning
and checking procedures that you may need to perform. This manual is intended for use by
qualied medical personnel only (state registered nurses, anaesthetists and doctors).
Keep this operating manual with the AlgiScan. It serves as a maintenance and repair manual.
Read the safety information in this manual carefully before using the AlgiScan.
Indications of use
The AlgiScan is a portable video pupillometer, intended to:
- Measure patients’ pupils quickly and easily,
- Evaluate the pupillary light reex of the patient following a photo-stimulation
- Evaluate nociceptive responses and the extent of analgesic response following an electrical
stimulation, in patients in the operating theatre, in recovery or in critical care
These patients are sedated and unable to communicate.
The usage mode for this device in non-continuous.
It provides the user with reliable, accurate and reproducible measurements. In a few
seconds the user has access not only to the measurement of the pupil diameter, but also
to the characteristics of its dynamic evolution: minimum, maximum, latency and speed of
evolution.
The size and reactivity of the pupil are considered as supplementary data which provide
more precise information on the analgesic condition and hypnotic state of the patient and his
pupillary light reex.
The use of AlgiScan allows to adjust the sedation dosage without replacing the usual practice
(clinical or pharmacological).
Two parts of the Algiscan may be in contact with the patient: the stimulation cable via the
appropriate electrodes and the eyecup.
Expected performance
The following features are the essential performance characteristics of the AlgiScan:
- Measure the average diameter of a patient’s pupil to an accuracy of +/- 0.1 mm (simulated
pupil of 3 mm diameter).
- Generate a white light stimulation with an intensity of 320 lux +/- 10%, and a duration of
1 sec +/- 0.1 sec.
- Generate electrical skin stimulation in the form of pulse train of 200μs, 100Hz, with an
amplitude between 0 and 60mA and a total duration between 0 and 8s.
Its characteristics are produced with an accuracy of +/- 10%.
Clinical Benets
The clinical benets of the AlgiScan are:
- A measurement of the patient’s analgesia level using their pupillary dilation reex.
- A precise measurement of the pupillary dilatation reex.
- A reproducible and quantitative measurement of the photomotor reex (Pupil Light Reex)
in neurological assessment.

User Manual AlgiScan 6
Important information about the use of AlgiScan
The AlgiScan compact video pupillometer is designed to be used by an authorised health
professional (anaesthetist, medical doctor or state registered nurse, anaesthetist nurse)
who has received special training in its use. The system, and all associated parameters, are
designed to be used on adult and paediatric patients in a hospital or health care facility to
monitor the responsiveness of their pupils to various stimuli.
The measurements performed by the AlgiScan on the pupillary reactivity of the patient can be
used to monitor the effects of certain anaesthetic products.
The interpretation of the results provided by the AlgiScan should always be subjected to
clinical judgement and compared with other clinical signs observed. We strongly advise
against relying solely on the results or values provided by the AlgiScan for monitoring sedated
or anaesthetised patients. The values provided by the AlgiScan should also be interpreted
with care when in the presence of certain products such as Barbiturate, Nitrous oxide, etc. It is
also necessary to interpret with care values measured on patients suffering from neurological
problems.
The AlgiScan conforms to the European directive covering medical devices and to the
regulations in force in the country of distribution.
For further information please contact IDMED, the manufacturer of the AlgiScan via their
website WWW.IDMED.FR or by post to the following address:
IDMED
Hôtel Technoptic
2 rue M. DONADILLE
13013 MARSEILLE
FRANCE
Phone : +33 (0)4.91.11.87.84
I Safety measures
INTRODUCTION
The user must read carefully the whole manual before operating the AlgiScan.
WARNING, CAUTION, NOTES
The terms Warning, Caution and Notes have precise meanings in this manual.
• WARNING warns against certain actions or situations likely to cause injury or death.
• CAUTION warns against actions or situations likely to damage the equipment, produce
inaccurate data or cancel a procedure, even if injury is unlikely.
• A NOTE provides useful information about a function or procedure.
EXPLANATION OF SYMBOLS
The symbols that may appear on the AlgiScan display are compiled and explained at the end
of this chapter.
Any serious incident occurring in connection with the device must be notied to the manu-
facturer and to the competent authority of the Member State or country in which the user and
the patient are established.

User Manual AlgiScan 7
I.1 Warnings
Explosion risk: do not use the AlgiScan in a ammable atmosphere or in places where
ammable anaesthetic products may accumulate.
The AlgiScan is not designed to operate in the environment of a SCANNER, M.R.I. or any
other appliance creating powerful magnetic elds.
The electrode wires, electrodes or connectors should not come into contact with other
conductors.
Never use the Electrical Stimulation of the AlgiScan while using high frequency surgical
appliances.
To reduce the risk of burns when using high frequency surgical appliances, do not place
the AlgiScan stimulation electrodes between the surgical site and the return electrode to
the electro surgery unit.
The connection to a patient at the same time as a high frequency surgery appliance can
cause burns at the points of contact of the AlgiScan electrodes and damage the appli-
ance.
Never use the AlgiScan simultaneously with the use of debrillation appliances.
The AlgiScan should never be connected continuously to electrodes positioned on the
patient but only for the period of measurement. Before and after the measurement, the
electric cable should be disconnected from the electrodes.
The AlgiScan must be connected to pacing electrodes or ECG electrodes supporting
voltages up to 300 Volts with a current of 60 mA. The contact area of the electrodes must
be greater than 1.8cm².
The power of the Electrical Stimulation causes nociceptive stimuli the intensity of which
should be appropriate to the patient’s analgesic level.
Never use the AlgiScan on a patient wearing a Pacemaker.
Never use the AlgiScan in the vicinity of short-wave or micro-wave therapy appliances.
Before using check that no other equipment, device or appliance is in contact with the
electrodes.
The electrodes may only be in contact with healthy, non-injured skin.
Before use, check the device, display and cable for damage. Never use the unit if any
defect or damage is found.
The AlgiScan in electrical stimulation mode (Tetanus, PPI) performs tetanus electrical
stimulus as well as increasing tetanus stimulations for a duration of 1 to 8 seconds. In
Tetanus and PPI mode, the AlgiScan provide electrical stimulation. When using a
monitoring system of neuromuscular blockade, the user should take care to wait 5
minutes after a Tetanus or PPI test before making a measurement of the patient’s level

User Manual AlgiScan 8
of neuromuscular blockade. In order not to bias the estimation of the measure of level of
neuromuscular blockade.
After taking any measurements, check the patient’s complete eyelid closure to protect
the eye from dryness and cornea alteration.
In order to prevent electromagnetic disturbance, keep minimum separation from RF
communication equipment of 30cm.
Use of this equipment adjacent to or stacked with other equipment should be avoided
because it could result in improper operation. If such use is necessary, this equipment
and the other equipment should be observed to verify that they are operating normally.
Any hardware or software modications to the device are prohibited.
Use of accessories, transducers and cables other than those specied or
provided by the manufacturer of this equipment could result in increased electromagnetic
emissionsorde-creasedelectromagneticimmunityofthisequipmentandresultinimproper
operation.
The electrodes will never be placed on the patient’s head or any other part of the body
that is judged to be potentially sensitive to electrical stimulation or may result in a risk of
injury to the patient.
Do not apply stimulation or place electrodes on the patient’s face and head (especially
the eyes, lips, entire neck). Do not position the electrodes on either side of the heart.
The use of the AlgiScan should not result in any signicant pressure of its eyecup on the
patient’s face but only putting it in contact. If too much pressure is applied, light marks
or red patches may appear on the skin in the contact area due to pressure points. They
should remain limited and not related to an injury.
I.2 Caution
Read this manual thoroughly and attentively before using the AlgiScan.
Do not autoclave the AlgiScan or any of its components or accessories other than the eyecup.
Never submerge the appliance or any of its components in liquid, or spray it or clean it with
liquid.
The AlgiScan and its components are not compatible with sterilisation processes by gas,
radiation (gamma or other), baths, steam or heat.
Follow the instructions for cleaning and disinfecting the AlgiScan given in the “Cleaning and
Disinfection” chapter.
The AlgiScan contains a lithium-ion battery. The AlgiScan battery must never in any
circumstances be removed, modied or replaced. Any intervention on the battery
presents a risk of re or explosion, only qualied technicians or IDMED company technicians
are competent to do such work.
After a long period of non-use (storage), recharge the battery of the AlgiScan for at least
4 hours before use. If the AlgiScan does not start when the device is picked up (motion

User Manual AlgiScan 9
detection), the AlgiScan must not be used and must imperatively undergo maintenance
operations.
Only qualied technicians are qualied to do repairs and maintenance, after obtaining
permission from IDMED.
The connection to a patient at the same time as a high frequency surgery appliance can
cause burns at the points of contact of the AlgiScan electrodes, and damage the appliance.
It should be noted that in all cases, in order to avoid any risk of corneal dryness, the use of
the device on the patient (open eye) should not exceed 60 seconds continuously. In order to
avoid any risk of corneal or retinal damage, the number of measurements performed on a
single patient should not exceed 10 per hour.
The AlgiScan’s users must take care not to be in contact with any other electrical appliances
when using the AlgiScan.
Before any stimulation the practitioner will evaluate the compatibility of the stimulation
intensity to the level of analgesia and sedation of the patient as well as the site of stimulation.
We strongly advise against placing the electrodes on the patient‘s thorax because of the
additional risks of cardiac brillation.
Never touch the electrodes during the stimulation phases. The electrodes are only surface
electrodes and are compatible with the application of electrical stimulation (CE marking
adapted).
Use only the accessories/components supplied by IDMED.
In order to prevent electrostatic shock, the device must be used in an electrostatic limited
environment (see Environment section).
Notice on Electromagnetic Compatibility (EMC) : This device generates, uses, and can
radiate radio frequency energy. If not set up and used in accordance with the instructions in
this manual, electromagnetic interferences may result.
The equipment has been tested and found to comply with the norm IEC60601-1-2 for medical
electrical equipment.
These limits provide reasonable protection against electromagnetic interferences when
operated in the intended environments (e.g. hospitals).
Known contraindications to use the AlgiScan: orbit structure damaged, surrounding soft tissue
oedematous, abraded skin.
User Manual AlgiScan 10
I.3 Explanation of symbols
Symbols are on the labels
Caution Serial Number
Indicates the necessity for
a separate processing from
household waste.
Marking for conformity with
the European directive cov-
ering medical appliances
Date of rst CE marking:
2010
Manufacturer IP 30
Protection class against
solid foreign bodies and
liquids.
Not protected against
liquids.
Refer to the user manual Type BF Applied Part
Catalog reference Direct Current DC
Prescription use only in the
USA Curtis-Straus Mark
(USA and Canada)
Manufacturing date Medical device
Qi Compliant Contains
FCC ID
Bluetooth module compli-
ant with the FCC regulation
part 15
Bluetooth module compliant
with Japanese regulation
MM
YYYY Manufacturing date,
Manufacturer
Symbols on AlgiScan screen
Main button Home button
Settings menu Previous step
Delete measurements or le Conrmation of the record-
ing of the measurement

User Manual AlgiScan 11
Pause activated Show records
Patient menu / Access to
patient records Add new patient’s le
Battery Charge level (full) Battery Charge level
(intermediary)
Battery Charge level
(empty) Mode selection
AlgiScan ready for taking
a measurement Measurement impossible
« Tetanus» mode « Flash » mode
« PPI©» mode « PRD » mode
Green electrode symbol.
Correct electrode
conduction level / skin.
Yellow electrode symbol.
Moderate electrode
conduction level / skin.
Red electrode symbol.
Non-functional electrical
stimulation, conduction
problem.
Grey electrode symbol.
Conduction problem, short
circuit, non-functional
electrical stimulation.
Left eye Right eye
Left eye/right eye
comparison Trend for the « Flash Mode »
Navigation arrows Time and date setting
Transport mode 3h / 24h data display
Bar code scanning function Data transfer menu /
Communication
Data transfer function
User Manual AlgiScan 12
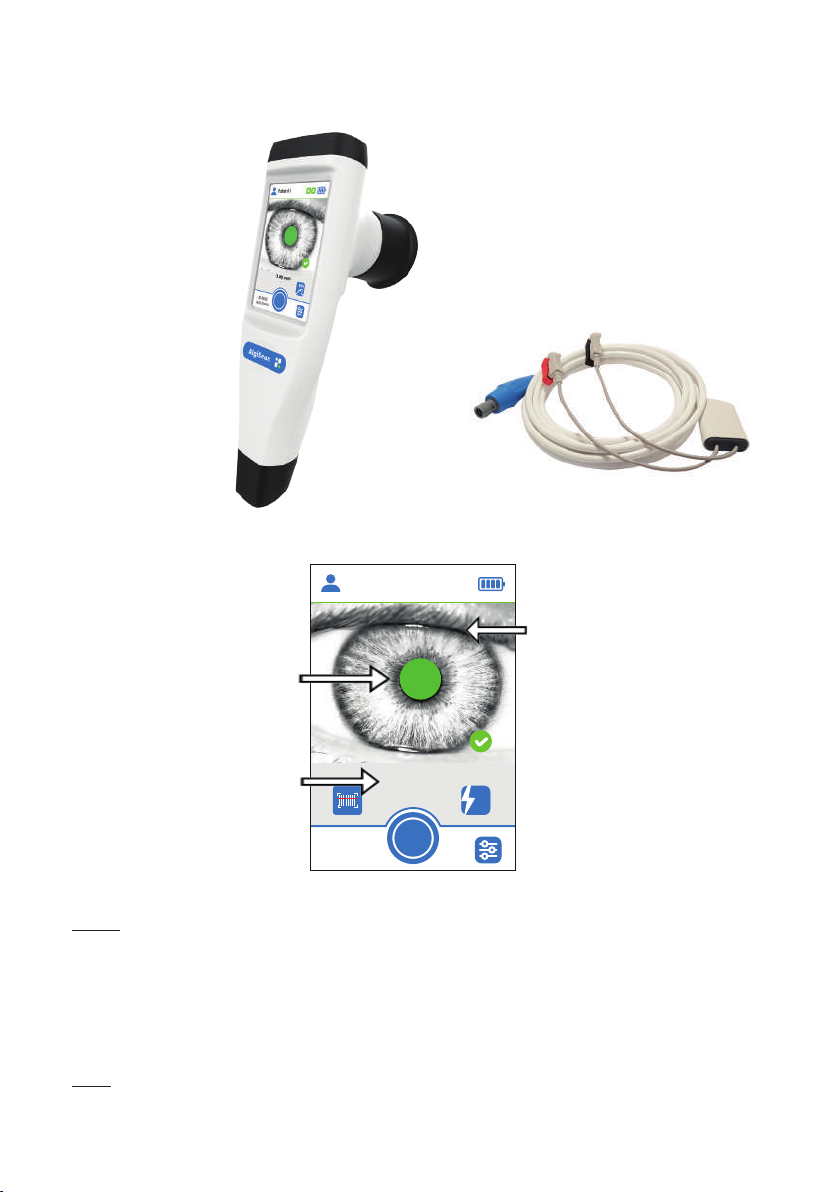
II General description
Overview of the AlgiScan and its accessories
Electrode wire
Display screen
Eyecup
Display screen
6.72 mm
10:30:12
14/01/2020
FL
Patient 1
Pupil correctly detected
(green surface)
Real-time
measured value
Video Image
Battery Charge Level
Main button
Note : display screen in the Flash mode (measurement mode used for measuring the
pupillary light reex)
Touch screen
The AlgiScan is designed in such a way that all controls are accessible with a simple
touch of a given area of the AlgiScan screen. This area is called the symbol or icon. The touch
screen is designed to work even if the operator is wearing gloves for the examination.
Note: the normal time to press the screen is approximately 1 to 60 seconds. Do not stay in
contact with the screen for more than 1 minute.
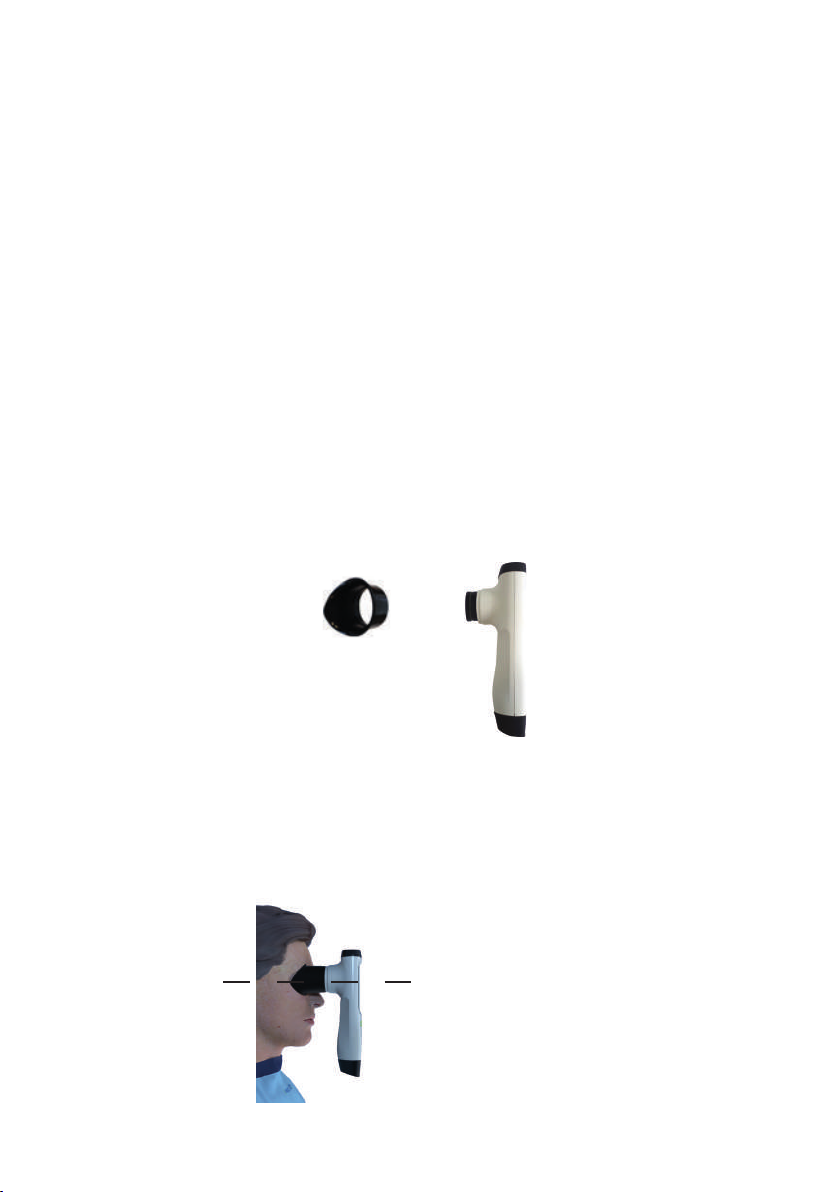
User Manual AlgiScan 13
General use
The AlgiScan can be used with a large number of different practices and protocols. It
can be used on all patients without any age limit. It allows exact measurement of the pupillary
light reex and the pupillary reex dilation. Hence the user should take care to conrm these
two reexes and their pertinence for every patient before using the AlgiScan.
It should be noted that in all cases, use of the appliance on the patient (with the
eye open) should not exceed 15 seconds continuously for the Tetanus, PPI and Flash modes.
For the PRD mode, the user starts the recording and stops it whenever needed. The maximum
recording time is 59sec. The number of measurements performed on the same patient should
not exceed 10 per hour.
Instantaneous measurement: an instantaneous measurement is a direct reading
of the pupil size by the user in real time on the AlgiScan screen.
Dynamic measurements consist of recording measurements under conditions
predened by the user by selecting one of the available modes of the AlgiScan. This type of
measurement will then give a series of results with gures and a curve representing the
variation in the size of the pupil. Algiscan offers 4 different modes (Flash, Tetanus, PPI and
PRD).
Fitting the positioning eyecup
The AlgiScan has a silicone eyecup so it can be positioned on the patient’s face.
Before use, the practitioner must clean this part. For more information on cleaning, please
refer to the chapter “CLEANING and DISINFECTION”. This eyecup is the only part in contact with
the patient. The eyecup is to be placed on the AlgiScan by simply pushing it onto the black
lens.
Installation and positioning of the AlgiScan
The correct positioning of the AlgiScan in relation to the lmed eye of the patient
enables more accurate measurements to be obtained. The black silicone end-piece on the
AlgiScan must be in contact with the upper and lower part of the eye socket bone without
pressing. In this way there will never be any pressure on the eye-ball itself. The appliance
should be held vertically and rmly so that it does not move in relation to the patient‘s head.
Correct positioning of the
AlgiScan
User Manual AlgiScan 14
Turning the device ON/OFF
The AlgiScan switches on automatically when the device is held in the hand
(movement detection).
Note: the device switches off automatically if it is not used for 2 minutes or if it is repositioned
on its charging station.
Patient Files / Measurements identication
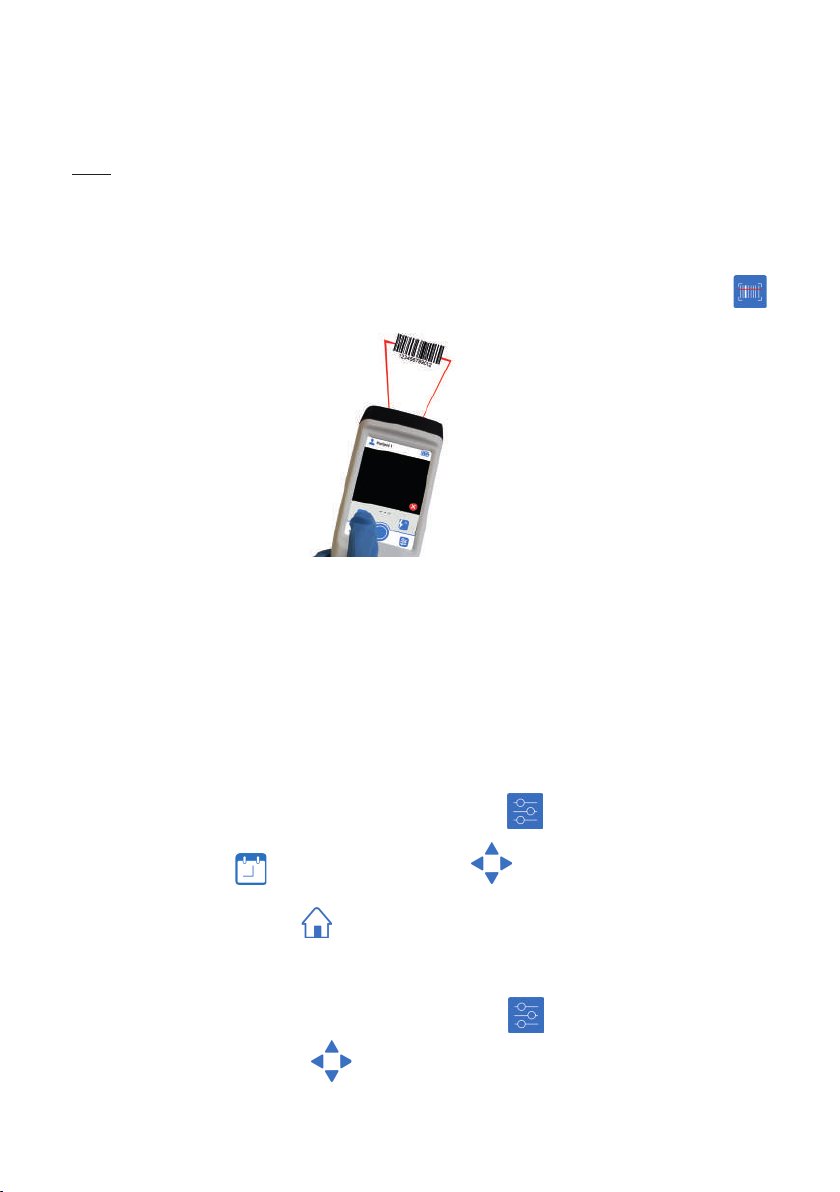
The AlgiScan has a barcode reader. Activation of the red light beam for reading
barcodes identifying the patient is done by pressing and holding the dedicated button
and directing the light beam towards the barcode.
Scan window
When a label is read, it creates a le with the label encoded ID number or retrieves
an existing le created by a previous scan of that same label.
The AlgiScan has also 20 pre-set les numbered 1 to 20. If there is no barcode
scanning, the user will either choose one of those les or create additional les numbered 21
and above to save the measurement results.
Settings
Setting the time on the AlgiScan
The AlgiScan contains a clock that is set at the factory. The operator can update the
date and time of the AlgiScan. To access the time setting and date change menu proceed as
follows:
• Select the “settings” menu by pressing the following icon
Then select this icon : . Use the navigation arrows to adjust the “date”, the “time” and
“format” of the date as desired.
By pressing the “Home” button , the changes will be automatically validated.
Changing the language
If necessary, the operator can change the language of the AlgiScan. To access the
menu for changing the language, follow the procedure below:
• Select the “Settings” menu by pressing the following icon
Then use the navigation arrows to select the desired “language”.
User Manual AlgiScan 15
Right Eye/ Left Eye Indicator
In the Flash mode, it is possible to identify the eye being lmed during measurement.
A pop-up window appears at the end of the measurement allowing the user to identify the
eye used with the following icons.
Transport Mode
The operator can set the AlgiScan to “transport mode.” This mode switches off the
unit for transport or storage. It prevents the unit from switching on every time a motion is
detected.
To access the “transport mode”, follow the procedure below:
• Select the “Settings” menu by pressing the following icon
• Then select the transport mode by pressing .
To deactivate the transport mode, position the device on its charging station. The device will
automatically switch on when it is removed from the station.
Data transfer / Communication
The user can select the mode and the data destination.
To access the data transfer menu, follow the steps below:
• Select settings menu by pressing on
• Then select data transfer by pressing on .
• Using the arrows select the transfer mode (PC/HL7)
• Connect your receiver module ; on PC (ref: NL-WDT) or HL7 (ref: IDM-GTW)
• Validate your choice by pressing on
Note: When activating the data transfer, the user must be at less than 1m (3ft) away from the
receiver.
When pairing, the type and identication (ID) at the bottom left of the screen must ll
automatically.
If not, get closer of the module and press again the following icon .
III Using the AlgiScan
Create or select a patient’s le
It is strongly advised to create a patient’s le or select an existing one to be able to
save the measurements.
To select an existing patient’s le:
• Press the icon “Patient File” and select the correct le with the left or right arrow
Or
• Scan the patient’s barcode that was previously used to create the le
To create a new patient’s le:
• Press the icon “Patient File” and select the icon “New patient’s le”. Numbering starts at 21.
Or
• Create a patient le by reading a barcode identifying a patient.

User Manual AlgiScan 16
Taking a measurement
Positioning of the device and detection of the pupil
Positioning the device on the patient’s face is an important step to obtain reliable
measurements (see: “Installation and positioning of the AlgiScan” section).
If the patient is conscious, ask him/her to keep his/her head straight, open the lmed
eye wide and look straight ahead without blinking. The other eye is closed.
If the patient is unconscious or not able to cooperate, the operator may need to
raise and/or lower the patient’s eyelids so that the pupil measured by the AlgiScan appears
completely unobstructed.
The patient’s pupil should be centered on the screen and totally in green color.
Only the pupil will be colored in the image displayed by the AlgiScan. If this is not the case,
reposition or position differently the AlgiScan.
It is important when measuring a very small pupil to position the AlgiScan so that the
white spots (reections of the infra-red diodes) do not deform the detected pupil contour. If
this is the case reposition the AlgiScan or position it differently.
NO
White spots deform the pupil YES
Correct positioning and
detection of the pupil
NO
Poor pupil detection
When the pupillometer is ready for a measure the following icon will appear at the
bottom right of the video image.
Note: when the pupil detection is not correct, the red icon will appear at the bottom right of the
video image. The pupillometer does not detect the pupil in the video image.
Do not move the AlgiScan when taking measurements
Taking a measurement
Once the device correctly positioned, the AlgiScan displays the green icon when the pupil
is detected. It is necessary to ensure that the size of the pupil is stable before taking the
measurement.
Start taking the measurement by pressing continuously on the main button until the
countdown at the bottom right corner of the screen reaches 0.
A beep will indicate the start and end of the measurement period. In “Flash” mode, at
the end of the measurement, the user can record the patient’s eye used for the measurement
(right or left).
Bilateral Pupil Exam: If a measurement on the other eye is done within 2 minutes of
the rst one, a comparison chart of the bilateral pupil exam will be displayed.
Hold function
When a mode (“Flash”, “Tetanus”, “PPI” or “PRD”) is selected, it is possible to freeze the analysed
image of the pupil by putting the AlgiScan on pause. To do this, press the video image for a
long time, which freezes the measured pupil value as well as the lmed image. Press again on
to deactivate the pause mode.

User Manual AlgiScan 17
Measurement protocols
The different modes will allow the pupil to be measured over a variable period of
time depending on the protocol chosen, with or without stimulation. In these different modes,
the operator starts measuring by pressing the main button continuously (the button is held
down throughout the measurement) after selecting the desired mode (“Flash”, “Tetanus”,
“PRD”, “PPI”). While recording, the seconds are counted down and displayed on the AlgiScan
screen. An audible beep indicates the start and nish of the recording (or measurement).
To ensure the quality of the results, the same precautions need to be respected as for an
instantaneous measurement:
• The pupil must be fully visible throughout the recording
• The pupil must be centred on the AlgiScan screen
• The green zone covering the pupil must remain homogeneous and stable.
« Flash » mode: The pupil reactivity is tested with a calibrated ash of light (photomotor
reex). Press the main button for 4 sec to start acquiring data. The stimulation is done with a
ash of light. The objective is to measure the pupillary reactivity to light.
« Tetanus » mode: It is a test of the patient’s pupil reactivity to an adjustable continuous
electrical stimulation (the reactivity of the patient’s pupil is recorded simultaneously to the
electrical stimulation).
This mode is used to measure the pupil reactivity to a standardized electrical stimulation.
Before starting the user selects the intensity of the electrical stimulation which will be applied
and makes sure it is innocuous for the patient.
(see Section : Selecting the measurement protocol)
« PRD » mode: It is a protocol to record the pupil size variation (the pupil is lmed with no
stimulation).
With this mode the variations of the pupil size are recorded during 60s in the course of medical
or surgery acts.
« PPI » mode: Evaluation of the patient’s level of sensitivity to nociception.
With this mode the patient’s level of analgesia is measured with the PPI scale. Before use, the
operator will check that the patient is in suitable conditions to withstand the different levels of
PPI mode stimulation and ensure that they are safe for the patient.
Before launching a measurement protocol, check that the selected protocol corresponds to
the desired one (“Flash”, “Tetanus”, “PRD” or “PPI”). If it is not the case, select the symbol of the
desired test (see the section: Selecting the Measurement Protocol).
The default protocol is shown on the right side of the screen. Before starting recording the
AlgiScan must be positioned on the patient and must detect the pupil correctly for at least 2
seconds to leave time to the pupil to adapt its size with the darkness.
Caution: before using the device, check the type of stimulation chosen, its intensity and
its innocuousness regarding the patient. For more information on these operations refer
to the following chapter.
User Manual AlgiScan 18
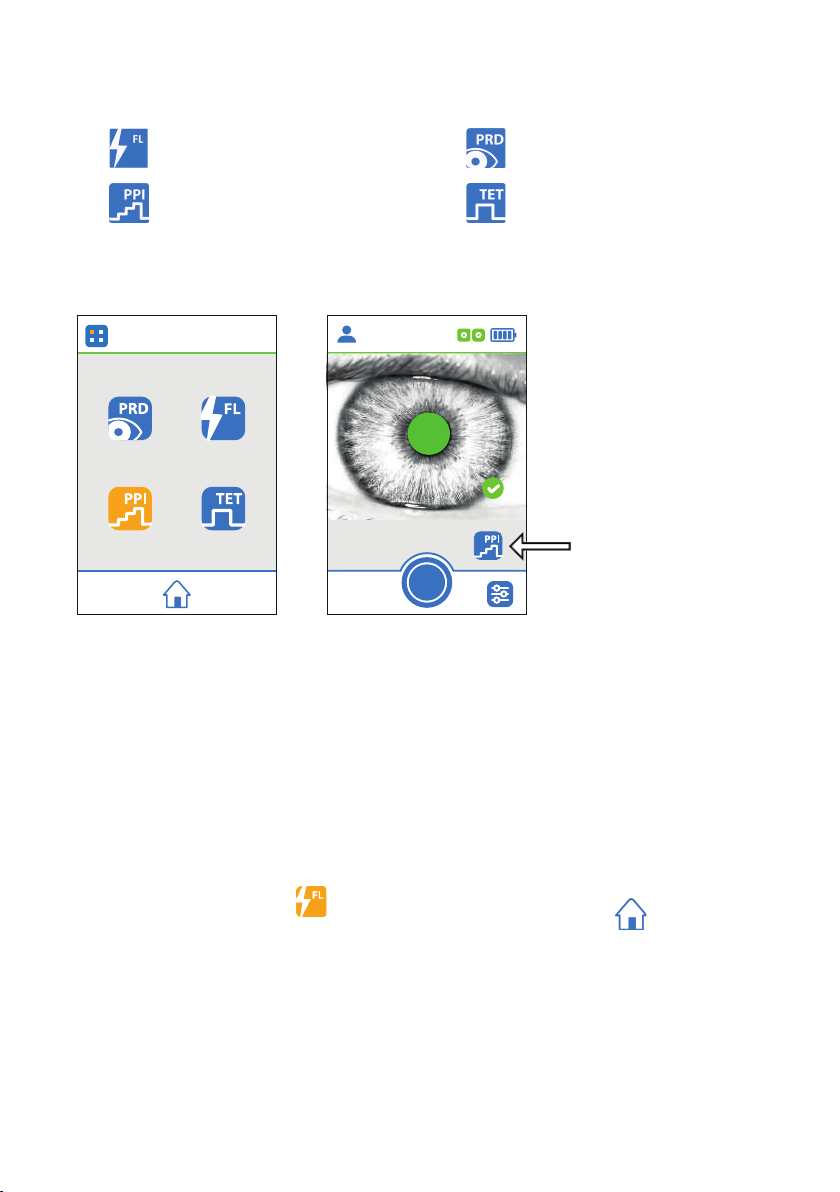
Selecting the Measurement Protocol
The AlgiScan uses four different symbols to show the mode in use.
“Flash” mode “PRD” mode
“PPI” mode “Tetanus” mode
By pressing the symbol displayed, you access the menu enabling you to select a mode. Once
in the “Mode Selection” menu, press the symbol of the desired mode. For Tetanus mode you
must specify the level of stimulation.
Menu « Mode selection »
60 mA
Mode of use
3.85 mm
10:30:12
14/01/2020
Patient 1
Display menu
selection page
“Flash” mode
The Light Flash protocol is used to check the efciency of the pupillary light reex.
The AlgiScan stimulates the patient’s pupil with a light ash. This lighting or light Flash has a
duration of 1 second. Its strength is xed at 320 Lux. The overall duration of the test is
4 seconds.
Selection of light stimulation
To select light stimulation and adjust its power:
• Press the symbol on the screen representing the mode used
• Once in the menu “mode selection” press the “ Flash “ symbol
• Flash mode icon selected
• Return to the measurement screen by pressing the following symbol
Starting a measurement with light stimulation
Once the ‘Flash’ mode has been selected, it is possible to start the measurement by
pressing the main button.
Before starting the measurement, the user must take care to position the appliance
for 2 seconds and check that the patient’s pupil is fully visible on the AlgiScan screen; the
eye not being lmed should be closed (for more information on the implementation of the
measurement refer to the chapter: “Taking a measurement”).
It is necessary to press and hold down the main button to perform the

User Manual AlgiScan 19
measurement. Then the AlgiScan emits a beep indicating the start of the measurement.
During this time, the AlgiScan should not be moved or the patient should close their eyelids. If
this is the case, a new measurement must be taken.
While the test is in progress (recording), the AlgiScan displays a countdown of the
remaining recording time (in seconds) during which it is necessary to keep the main button
pressed. A nal beep indicates the end of the measurement.
“PRD” mode
This mode allows to measure (record) the pupil size changes during 60s at
maximum. This operating mode does not require for the stimulation cable to be connected
to the device.
The AlgiScan does not generate any stimulation during this test.
The interest of this mode is to accurately measure pupil reactions during nociceptive stimuli
(surgical or medical care) via the pupil reex dilation. The pupil dilation thus measured makes
it possible to quantify the intensity of the stimuli and to evaluate their impact on the patient
and their actual opioid needs.
Starting “PRD” mode
• Select the desired patient le.
• Select PRD mode (icon: ) via the menu “mode selection”.
• Position the device on the patient’s eye.
• Return to the measurement screen by pressing the following symbol .
• Check as pupil is correctly detected and coloured in green on the screen.
• Start recording by pressing the main button
• Press and hold the main button for the duration of the recording.
Recording stops automatically at 60s or when the user releases the main button.
The result is not valid if the recording time is less than 2 seconds, and will not be displayed.
“Tetanus” mode
This protocol measures the dilation of the pupil of a patient subjected to
electrical stimulation. The duration of this test is 10s. It is essential to take into account the
patient’s analgesic and/or sedation level to determine the optimal stimulation intensity. In
case of doubt we advise using the lowest intensity stimulation possible.
Characteristics of electrical stimulation
The electrical stimulation generated by the AlgiScan is the stimulation regularly used by NMT
Monitor and “peripheral nerve stimulators”. This stimulation is called “Tetanus 100Hz”.
The characteristics are detailed in the «Technical Specications» section.
It is a nociceptive stimulation that should be adapted in intensity to the patient’s analgesic
level.
It is common practice to retain the following stimulation intensity thresholds :
• Non-sedated person, below 10mA
• Normally sedated person, 0 to 40mA (patient in ICU or operating theatre)
• Heavily sedated person 0 to 60mA(ICU or Operating theatre)
Caution: these values are only observations and should in no circumstances serve as a
prescription. Only a qualied practitioner can determine the appropriate stimulation level
to be applied to the patient.

User Manual AlgiScan 20
If necessary, the tetanus stimulation stops as soon as the user releases the main button.
The result is not valid if the recording is stopped less than 2 seconds after the end of the
electrical stimulation, and will not be displayed.
Selection of electrical stimulation and its intensity
To select electrical stimulation and adjust its intensity:
• Press the symbol on the screen representing the mode used
• Select “Tetanus” mode.
• Select the intensity of the electrical stimulation by pressing the navigation arrows .
60 mA
Choice of intensity
60
Mode of use
Select the stimulation intensity by
pressing the navigation arrows
until the desired value is displayed.
• Conrm your selection by pressing the following symbol
Note: To exit the pop-up window or cancel the intensity selection, press the following symbol
Once the intensity has been adjusted, the ‘selection mode’ screen is being displayed again.
The user can then visually check the selected intensity.
In the example above, the user has selected a 60 mA electrical stimulation.
The user returns to the measurement screen by pressing the following symbol
The AlgiScan indicates continuously the level of conduction of the patient’s electrodes.
3.85 mm
10:30:12
14/01/2020
Patient 1
60 mA
Electrical stimulation
selected with a current
of 60 mA
Verication of the level of
conduction of the electrodes.
In this example the conduc-
tion is OK (green).
Other manuals for Algiscan
1
Table of contents
Other Idmed Medical Equipment manuals
Popular Medical Equipment manuals by other brands
ThermoTek
ThermoTek VascuTherm 4 user manual

Electronique du Mazet
Electronique du Mazet ECHODIA Babyscreen Instructions for use and Technical description

Boscarol
Boscarol OB 2012 FA user manual

ORTHOSERVICE RO+TEN
ORTHOSERVICE RO+TEN castop 750 quick start guide

KAP Medical
KAP Medical K-1 Series operating instructions

Olympus
Olympus ESG-410 Quick reference guide

dymax
dymax BlueWave 200 Quick start instructions

B. Braun
B. Braun OUTLOOK ES Series Operator's manual
BIOTRONIK
BIOTRONIK Pulsar-18 T3 Instructions for use

ultraMEDIC
ultraMEDIC SAN-0090 operating manual

College Park
College Park Truper Technical instructions

Handicare
Handicare SystemRoMedic Vega505EE user manual