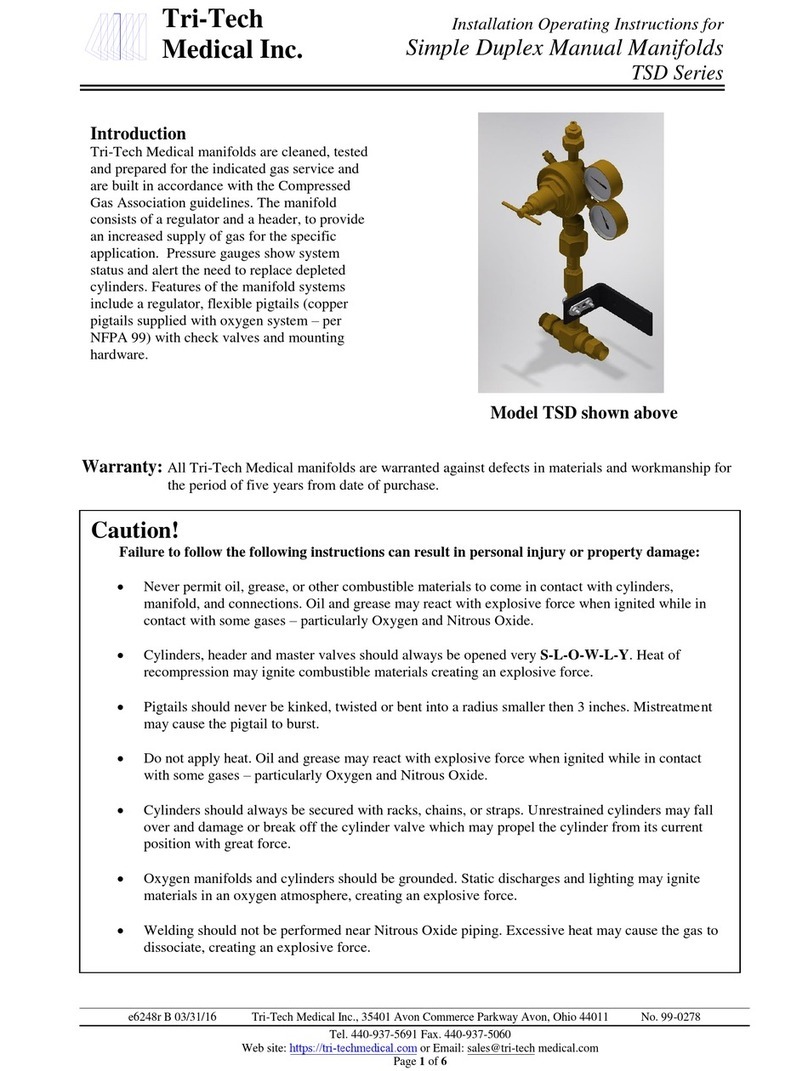
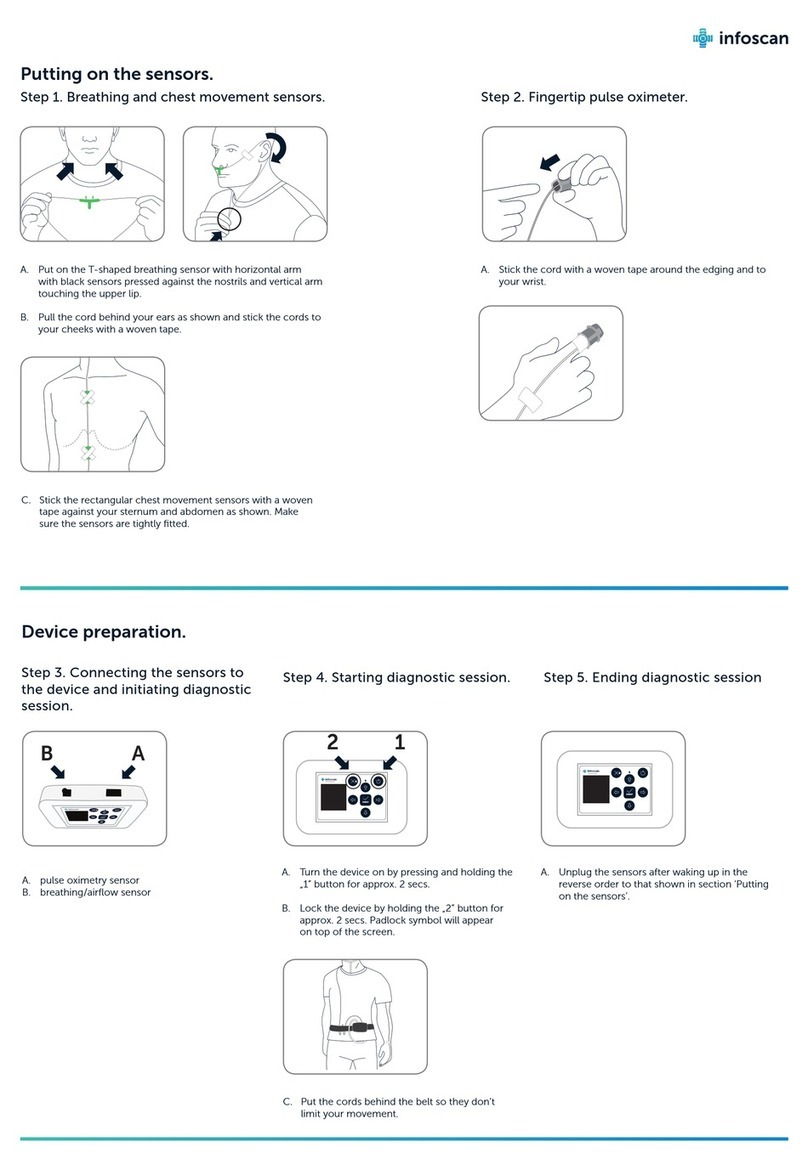
M y L a b –S A F E T Y A N D S T A N D A R D
No confirmed biological effects on patients or instrument operators caused by
exposure at intensities typical of present diagnostic ultrasound instruments have
been reported. Although the possibility exists that such biological effects may be
identified in the future, current data indicate that the benefits to patients deriving
from the prudent use of diagnostic ultrasound outweigh the risks, if any, that may
be present.
The ALARA (AsLow AsReasonably Achievable) principle is the guideline for
prudent use: during an exam, the user should use for the shortest duration the least
amount of acoustic output to obtain the necessary clinical information for
diagnostic purposes.
Ultrasound Bioeffects
Although diagnostic ultrasound has an excellent history of safety, it has been
known for a long time that ultrasound, at certain levels, can alter biological systems.
The AIUM Bioeffects Committee describes two fundamental mechanisms by
which ultrasound may induce biological effects: non-thermal or mechanical
mechanisms1 and thermal effects.
Non-thermal bioeffects, also referred to as mechanical bioeffects, seem to be
caused by the tissue alternate expansion and contraction induced when ultrasound
pressure waves pass through or near gas. The majority of these non-thermal
interactions, also known as cavitation, deal with the generation, growth, vibration,
and possible collapse of microbubbles within the tissue. The occurrence of
cavitation depends on a number of factors, such as the ultrasonic pressure and
frequency, the ultrasonic field (focused or unfocused, pulsed or continuous), the
nature and state of the tissue and boundaries. Mechanical bioeffects are a threshold
phenomenon, occurring only when a certain level of output is exceeded. However,
the threshold level varies depending on the tissue. The potential for mechanical
effects is thought to increase as peak rarefactional pressure increases, but to
decrease as the ultrasound frequency increases.
Although there have been no adverse mechanical bioeffects in humans from
diagnostic ultrasound exposure, it is not possible to specify thresholds at which
cavitation will occur in mammals.
Thermal bioeffect is the rise in temperature of tissue when exposed to acoustic
energy. The acoustic energy is absorbed by body tissue; absorption is the
conversion of this energy into heat. If the rate of energy deposition in a particular
region exceeds the ability to dissipate the heat, the local temperature will rise. The
rise in temperature will depend on the amount of energy, the volume of exposure,
and the thermal characteristics of the tissue.
1 American Institute of Ultrasound in Medicine Bioeffects Committee, Bioeffects Considerations for the
Safety of Diagnostic Ultrasound, J. Ultrasound Med., 1988, 7 Suppl.
M E C H A N I C A L
B I O E F F E C T S
“Cavitation” phenomenon
T H E R M A L
B I O E F F E C T
Rise in temperature of
tissue exposed to acoustic
energy.