3
Instructions for Use
Carrier
Table of Contents
Table of Contents
For your safety ...................................................................................................................................................................................................................................................................................................................................................... 4
Intended purpose and indications .................................................................................................................................................................................................................................................................................... 5
Contraindications ........................................................................................................................................................................................................................................................................................................................................... 5
Component definitions ........................................................................................................................................................................................................................................................................................................................... 5
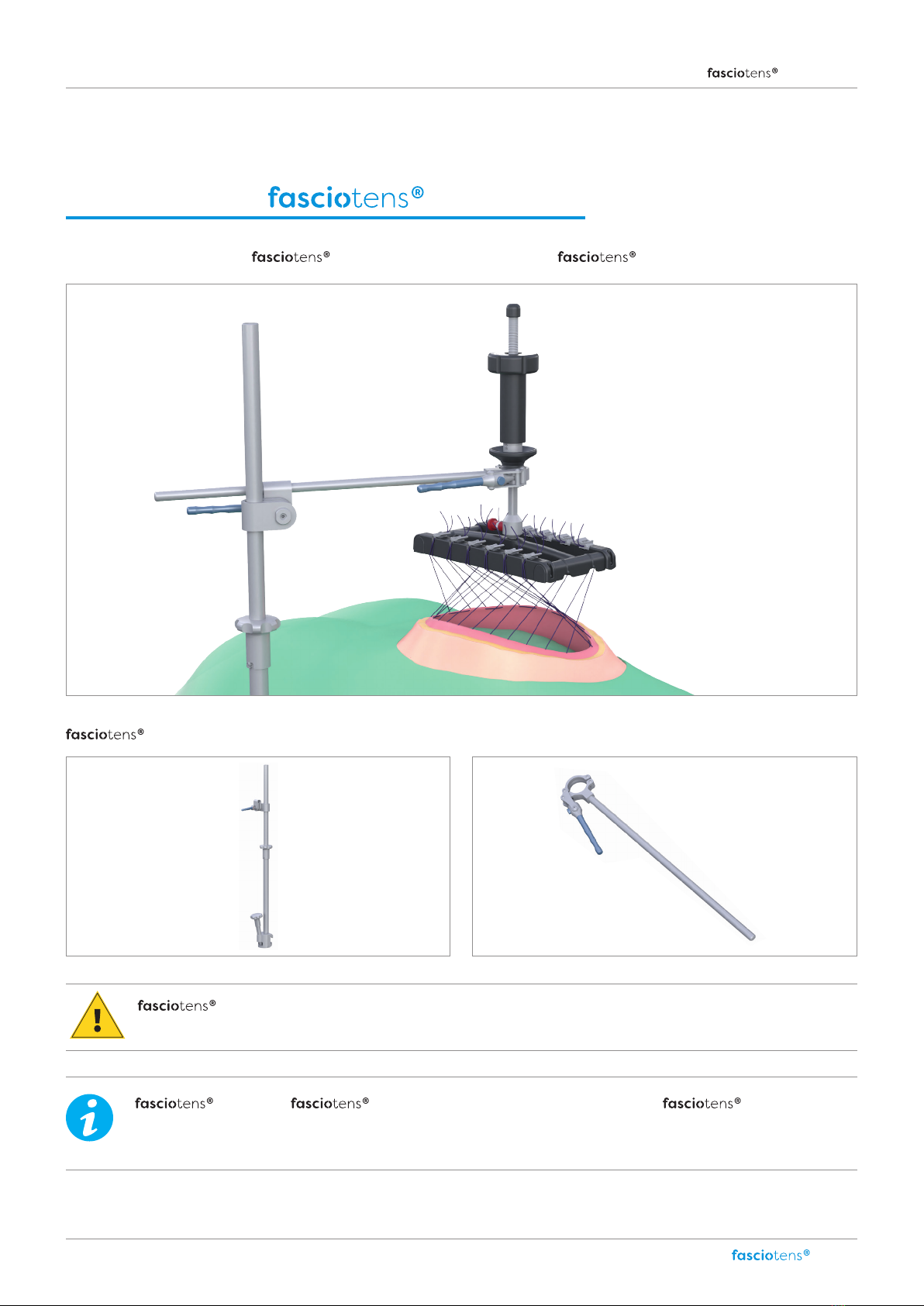
Product structure Carrier (HC010) ................................................................................................................................................................................................................................................ 6
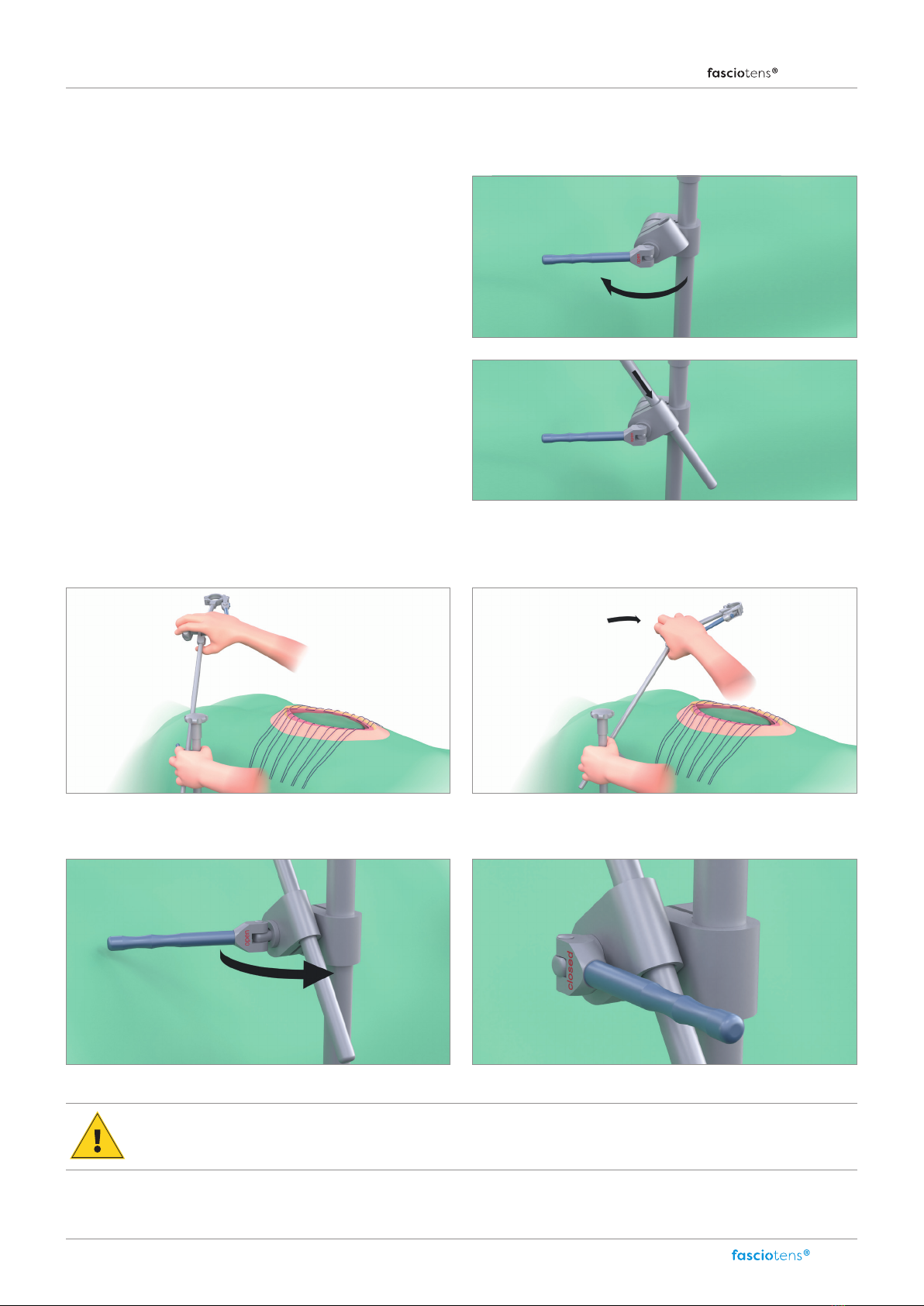
Assembly Carrier (HC010) ........................................................................................................................................................................................................................................................................ 7
Product structure Carrier (HC020) ............................................................................................................................................................................................................................................ 10
Product assembly Carrier (HC020) ....................................................................................................................................................................................................................................... 11
Combination with Hernia .................................................................................................................................................................................................................................................................... 14
Processing instructions Carrier .................................................................................................................................................................................................................................................... 15
Service life ......................................................................................................................................................................................................................................................................................................................................................................15
Preparation ..................................................................................................................................................................................................................................................................................................................................................................15
Cleaning ..........................................................................................................................................................................................................................................................................................................................................................................16
Sterilisation ...............................................................................................................................................................................................................................................................................................................................................................17
Final instructions ..............................................................................................................................................................................................................................................................................................................................................17
Storage instructions .....................................................................................................................................................................................................................................................................................................................................18
Maintenance ............................................................................................................................................................................................................................................................................................................................................................18
Repairs ...............................................................................................................................................................................................................................................................................................................................................................................18
Template for returns ................................................................................................................................................................................................................................................................................................................................ 19
Warnings ..................................................................................................................................................................................................................................................................................................................................................................... 20
Warranty ...................................................................................................................................................................................................................................................................................................................................................................... 20
Support .......................................................................................................................................................................................................................................................................................................................................................................... 20