fasciotens Carrier User manual
Instructions for use
www.fasciotens.de

3
Instructions for use
Carrier
Introduction
Dear customer,
Thank you for choosing the
fasciotens®Carrier. fasciotens® products provide the highest quality, safety and state-of-the-art
technology.
This product was developed in partnership with practising surgeons.
To take full advantage of this product’s capability and to ensure its successful application, please read the Instructions for Use
carefully and use the product as instructed. Always follow standard safety precautions for general occupational safety, your
specic SOPs and all relevant regulatory requirements. We will not assume liability for any damage arising from improper use
or use contrary to its intended purpose or incorrect handling.
Any serious incidents that occur in connection with the product must be reported immediately to fasciotens GmbH
and the responsible national authority.
This medical device is reserved for use by medical professionals only. Please make sure that all persons using this
product only do so aer having read and understood the Instructions for Use.
Please keep the Instructions for Use in a safe place; you may want to reread them at a later date.
Company address:
fasciotens GmbH
Moltkeplatz 1
D-45138 Essen
Tel. +49 (0)201 99 999 630
Fax +49 (0)201 99 999 639
Email: info@fasciotens.de
Website: www.fasciotens.de

4
Instructions for use Carrier
Table of Contents
Table of Contents
For your safety ............................................................................................................................................................................................................................................ 5
Intended purpose, indications and contraindications.................................................................................................................................................... 6
Component denitions .......................................................................................................................................................................................................................... 6
Product design fasciotens®Carrier (HC020) ........................................................................................................................................................................... 7
Product assembly fasciotens®Carrier (HC020) ..................................................................................................................................................................... 8
Combination with fasciotens®Hernia .......................................................................................................................................................................................... 11
Processing fasciotens®Carrier ........................................................................................................................................................................................................... 12
Service life ......................................................................................................................................................................................................................................................... 12
Preparation ....................................................................................................................................................................................................................................................... 12
Cleaning ............................................................................................................................................................................................................................................................ 13
Sterilisation ..................................................................................................................................................................................................................................................... 15
Final instructions ......................................................................................................................................................................................................................................... 16
Storage instructions .................................................................................................................................................................................................................................. 16
Maintenance ................................................................................................................................................................................................................................................... 16
Template for returns ................................................................................................................................................................................................................................. 17
Repairs................................................................................................................................................................................................................................................................. 18
Disposal............................................................................................................................................................................................................................................................... 18
Warranty............................................................................................................................................................................................................................................................. 18
Support................................................................................................................................................................................................................................................................ 18
Symbols used.................................................................................................................................................................................................................................................. 19

5For your safety
Instructions for use
Carrier
For your safety
Please observe the Instructions for Use
Any application or handling of the medical device requires precise knowledge and observation of the Instructions for Use.
The product may only be used for the purpose described.
Statements of particular importance are agged as follows in the Instructions for Use:
Liability for proper function and damage
Any liability for damage caused by use of the product is always transferred to the operator or user, insofar as the product is
used by persons who do not belong to the relevant professional groups, who do not have the relevant qualications required
to operate the product or who have not received proper instruction in its use. In addition, liability is transferred to the user
in case of improper use or the product is used inappropriately.
Prior to use, the product is to be inspected to ensure it is intact and not damaged in any way.
The warranty and liability conditions of the terms and conditions of sale and delivery of fasciotens GmbH are not extended
by any previous or subsequent references.
Please ensure that the Instructions for Use are accessible at all times and that they are read and understood.
Warning!
This is a Warning alerting you to risk situations and dangers.
Ignoring such a warning may lead to life-threatening situations.
Warnings must be observed under all circumstances.
Information!
This is information about specific features that need to be considered under all
circumstances.
6
Instructions for use Carrier
Intended purpose, indications and contraindications/component denitions
Intended purpose, indications and contraindications
Intended purpose
The intended use for the fasciotens®Carrier is as a holding device for fasciotens products before, during and aer surgical pro-
cedures. fasciotens®Carrier is a class I medical device.
The product is intended exclusively for human medical purposes and is used during surgery.
The product is approved for use in combination with fasciotens®Hernia.
The combination with any products other than fasciotens®Hernia has not been verified and validated by
the manufacturer. The intended purpose does not include such a combination and is the responsibility
of the user.
Indications
• Combination with
fasciotens®
products
• Combination with operating tables or standard rails
Contraindications
• No suiciently stable fastening rail
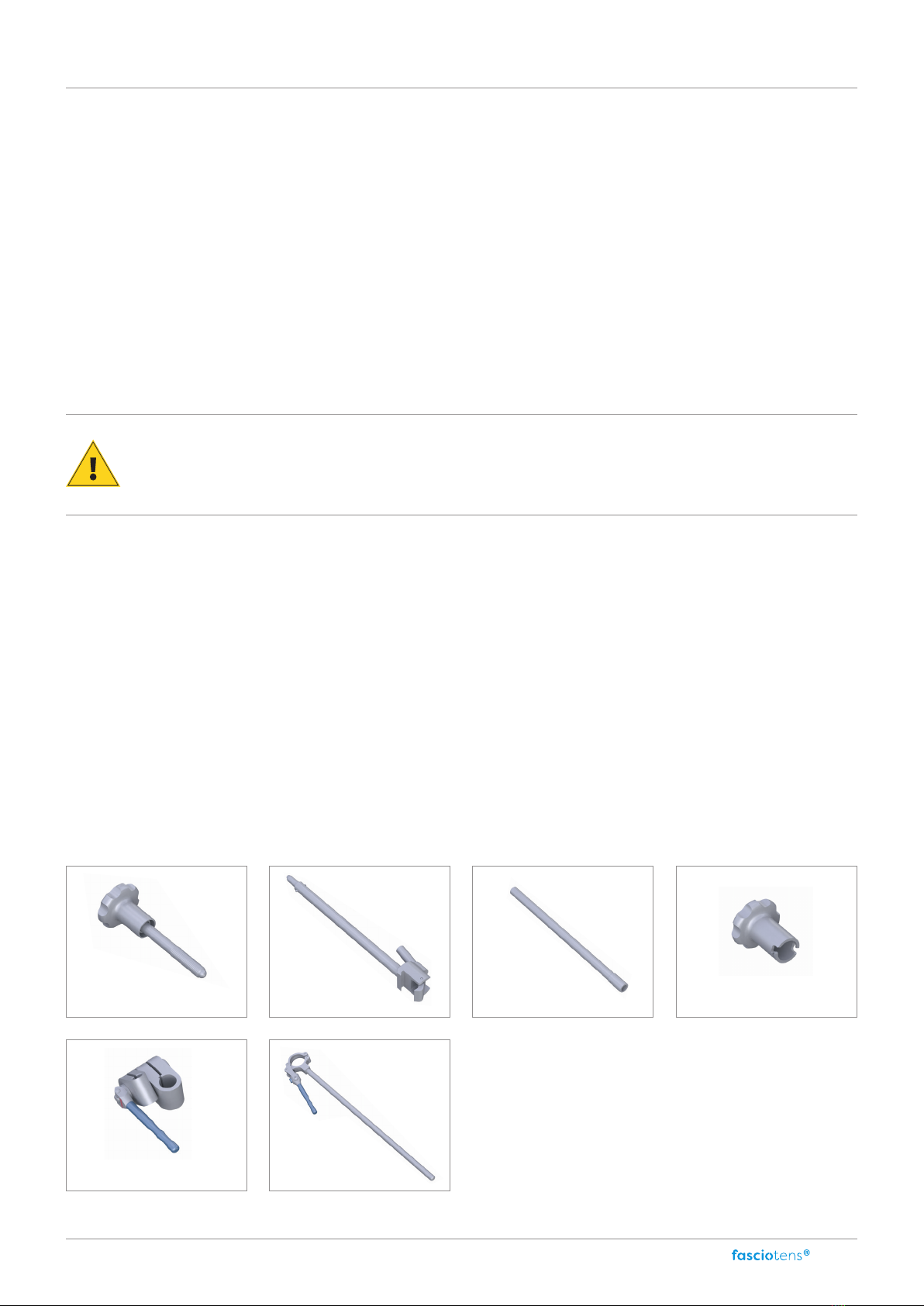
Components
Screw head post
Post
base (P1)
Post
extension (P2)
Screw head
extension (P3)
Eccentric handle (P4) Articulated arm
7
Instructions for use
Carrier
Product design fasciotens®Carrier HC020
Product design fasciotens
®
Carrier HC020
fasciotens®Carrier
and
fasciotens®Hernia
may only be used in a sterile condition.
fasciotens®Carrier
is supplied in a
non-sterile condition by the manufacturer and must be sterilised in the hospital before each use in the operating
room. Please follow the processing instructions. Please follow the storage instructions for the product.
fasciotens®Carrier
must be sterilised before use by the CSSD according to the processing instructions.
Post HC021 Articulated arm HC022
fasciotens®Carrier
(HC020) consists of the following modules:
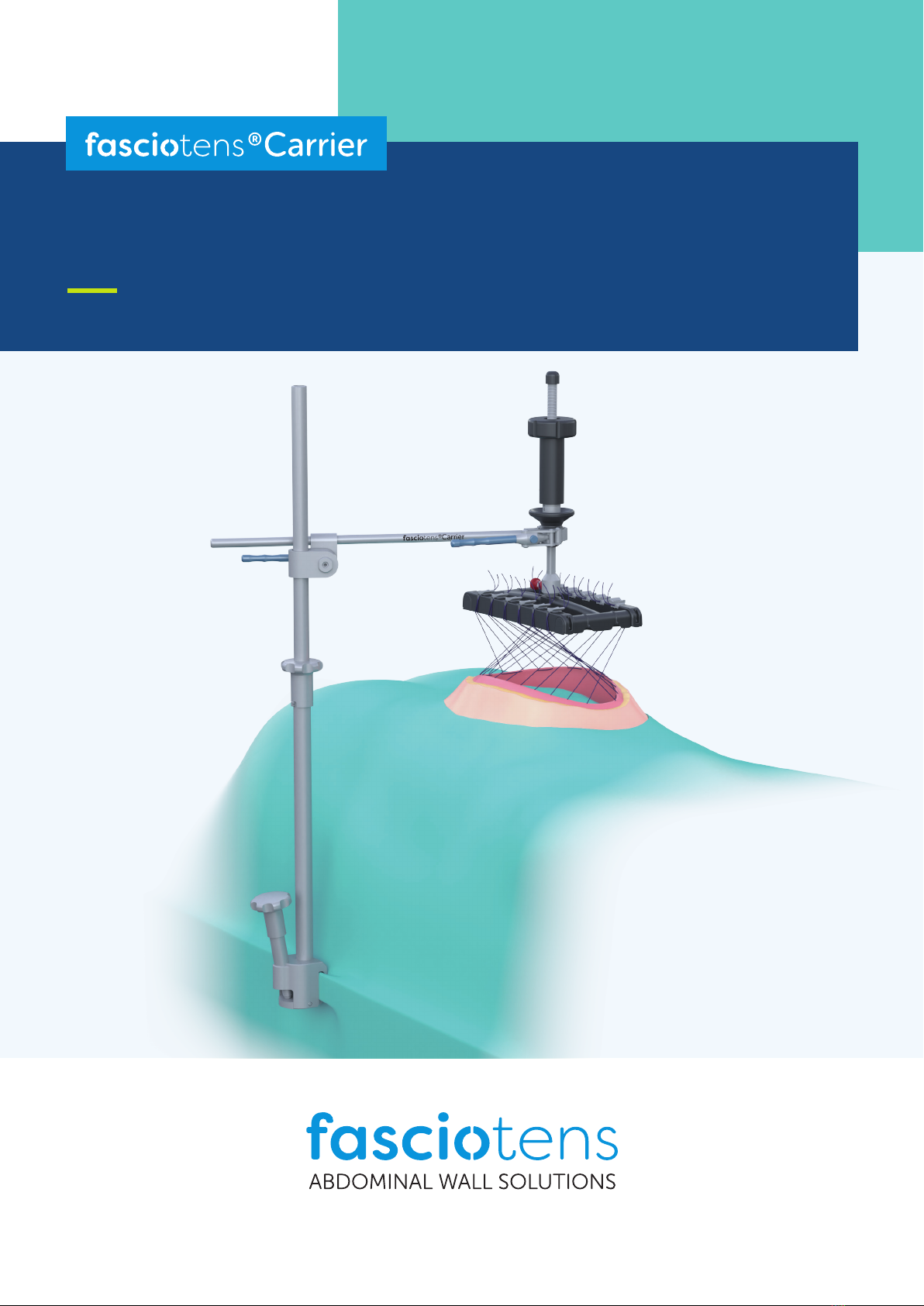
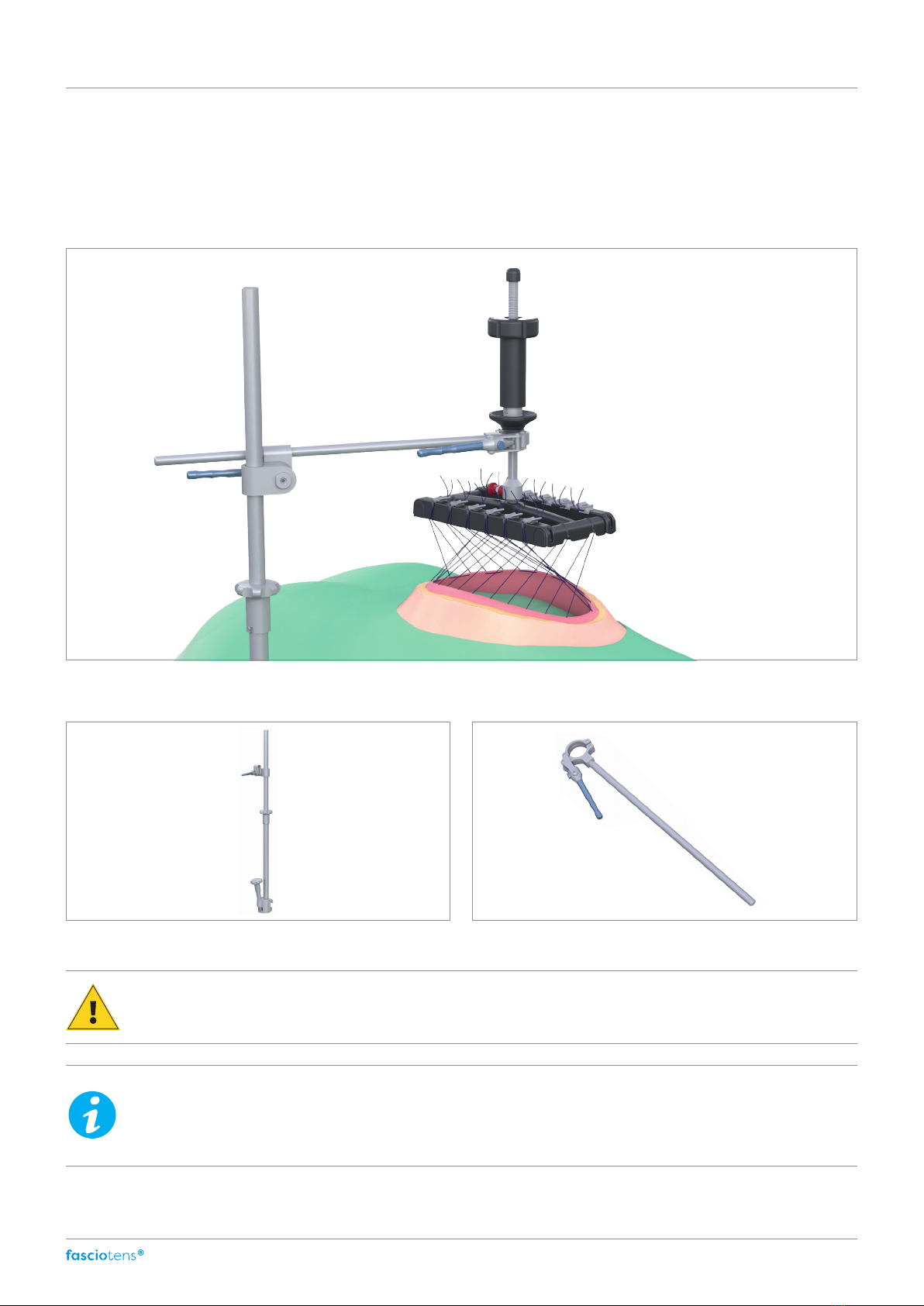
The gure below shows how the product
fasciotens®Hernia
is used in combination with
fasciotens®Carrier.

8
Instructions for use Carrier
Product assembly
fasciotens®Carrier HC020
Assembly of the product
fasciotens®Carrier can be attached to all operating tables that have a standard rail. The post is mounted above the sterile
cover on the operating table. The position of the post can be determined by the user but should not obstruct the surgeon.
Make sure that the product has been sterilised beforehand according to the processing instructions.
1. Remove the components from the tray and place them on the instrument
table. Make sure that the clamp opening at the lower end of the post is fully
open.
2. Turn the screw head into the hole provided for this purpose at the lower end
of the central post.
3. Place the post on the standard rail of the operating table.
4.
Lock the post base (P1) on the standard rail of the
operating table by turning the screw head clockwise.
5. Guide the post extension (P2) onto the upper end of
the part of the post attached to the operating table.
Make sure that the post is properly attached and that there are no
objects in the way which would prevent/impair proper attachment
(e.g. patient blanket, catheter, ECG cable).
The operating table cover should have no more than 2 layers.
Check that it is firmly in place on the operating table.

9
Instructions for use
Carrier
Product assembly fasciotens®Carrier HC020
6. Guide the screw head extension (P3) with the opening onto the post extension and connect both parts of the post
by turning the screw head.
7. Guide the eccentric handle (P4) onto the top of the
post extension and move it to the desired position.
8. Insert the articulated arm into the open eccentric handle.
Check that both modules are firmly locked.
Always hold the eccentric handle with one hand to
prevent it from falling suddenly when not locked.

10
Instructions for use Carrier
Product assembly
fasciotens®Carrier HC020
9. Align the articulated arm over the patient based on the defect and the abdominal circumference.
10. Secure the articulated arm in the eccentric handle by ipping the clamping lever.
The word "closed" will now be visible on the eccentric lock.
Before each use, check that the product is intact and make sure that the post and the articulated arm are attached
in a secure and sterile manner.
The post and the articulated arm can cause pressure damage. Make sure that there is always suicient space
between the patient and the articulated arm.
This manual suits for next models
1
Table of contents
Other fasciotens Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual














