Fluidigm Juno User manual

PN 100-7074 D1
Genotyping
with Juno
Getting Started Guide

Genotyping with Juno Getting Started Guide
2
For technical support visit fluidigm.com/support.
Japan +81 3 3662 2150 | [email protected]
China (excluding Hong Kong) +86 21 3255 8368 | [email protected]
For Research Use Only. Not for use in diagnostic procedures.
Information in this publication is subject to change without notice. It is
Fluidigm policy to improve products as new techniques and components
become available. Therefore, Fluidigm reserves the right to change
specifications at any time. Every effort has been made to avoid errors in
the text, diagrams, illustrations, figures, and screen captures. However,
Fluidigm assumes no responsibility for any errors or omissions. In no
event shall Fluidigm be liable for any damages in connection with or
arising from the use of this publication.
Patent and Limited License Information
Fluidigm products are covered by issued and pending patents in the
United States and other countries. Patent and limited license information
is available at fluidigm.com/legalnotices
Limited Use License to Perform Preamplification with Fluidigm IFCs
A license to use Thermo Fisher Scientific’s patented preamplification
method workflows involving a Fluidigm integrated fluidic circuit (IFC) can
be obtained (i) with purchase of a Fluidigm IFC from Fluidigm Corporation
or (ii) by a separate license from Thermo Fisher Scientific. For licensing
Limited Digital PCR License
A license to use Thermo Fisher Scientific’s patented digital PCR method
in all fields other than in the Sequencing Field, the Mass Spectrometry
Field, and the Prenatal Field in workflows involving a Fluidigm IFC can be
obtained (i) with purchase of a Fluidigm IFC from Fluidigm Corporation or
(ii) by a separate license from Thermo Fisher Scientific. For licensing
Trademarks
Fluidigm, the Fluidigm logo, Biomark, EP1, Juno, and SNP Type are
trademarks or registered trademarks of Fluidigm Corporation in the
United States and/or other countries. All other trademarks are the sole
property of their respective owners.
For EU's WEEE directive information, go to fluidigm.com/compliance
© 2017 Fluidigm Corporation. All rights reserved. 09/2017

Genotyping with Juno Getting Started Guide 3
Contents
About This Guide 4
Purpose 4
How to Use This Guide 5
Safety Alert Conventions 5
Safety Alerts for Chemicals 5
Safety Alerts for Instruments 5
Safety Data Sheets 6
Getting Started 7
Workflow 7
Best Practices 7
Related Documentation 7
Chapter 1: Product Information 8
Required Kit Contents 8
TaqMan Assay Kit 8
SNP Type Assay Kit 9
Required Reagents 10
TaqMan Assays 10
SNP Type Assays 10
Suggested Reagents 10
Required Consumables 11
Required Equipment 11
Suggested Equipment 11
Required Software 12
IFC Type and Related Scripts 12
Chapter 2: Genotyping with the
Juno 96.96 Genotyping IFC Using
TaqMan Assays 13
Prepare Assay and Sample Mixes 13
Prepare the Primer Pool for Preamplification 13
Prepare 2X TaqMan Assays for Genotyping 14
Prepare the Assay Mix 14
Obtain the Minimum Required Genomic DNA 14
Prepare the Sample Mix 15
Load and Run the IFC on Juno 16
Perform Genotyping Analysis on the Samples 19
19
Chapter 3: Genotyping with the Juno
96.96 Genotyping IFC Using SNP
Type Assays 20
Prepare Assay and Sample Mixes 20
Prepare the 200 nM Primer Pool for
Preamplification 20
Prepare 2X SNP Type Assays 21
Prepare the Assay Mix 22
Obtain the Minimum Required Genomic DNA 22
Prepare the Sample Mix 23
Load and Run the IFC on Juno 24
Perform Genotyping Analysis on the Samples 27
27
Appendix A: Suggested Kits 28
TaqMan Assay Kit 28
SNP Type Assay Kit 28
Suggested Reagents to Use with TaqMan
Assay and SNP Type Assay Kits 29
Appendix B: Safety 30
General Safety 30
Instrument Safety 30
Electrical Safety 31
Chemical Safety 31
Disposal of Products 31

Genotyping with Juno Getting Started Guide
4
About This Guide
CAUTION ABBREVIATED SAFETY ALERTS. Hazard symbols and hazard
types specified in procedures may be abbreviated in this document. For
complete safety information, see the safety appendix on page 30.
Purpose
This guide describes how to perform genotyping of low-concentration DNA with
the Juno™96.96 Genotyping IFC (integrated fluidic circuit) on the Juno™ system.
This is possible through advanced microfluidics technology that integrates
preamplification and genotyping reactions of up to 96 samples and 96
genotyping assays in a single workflow on an IFC.
The IFC produces 9,216 genotypes in less than three hours using a simple
workflow with minimal hands-on time. Samples are loaded into individual inlets of
the Juno 96.96 Genotyping IFC, then distributed across multiple reaction
chambers in nanoliter-volume aliquots. With high-quality samples, detecting the
specific targets requires thermal cycling for preamplification and PCR for
genotyping on the instrument.
After genotyping is performed on the Juno system, the IFC is scanned on the
EP1™system or the Biomark™HD system to collect genotyping data for later
analysis.
Genotyping with Juno Getting Started Guide
About This Guide
How to Use This Guide
5
How to Use This Guide
The chapters in this guide are organized according to assay type. Refer to the
appropriate chapter to run the Juno 96.96 Genotyping IFC on the Juno system.
For detailed instructions on instrument and software operation, refer to the Juno
System User Guide (PN 100-7070).
Safety Alert Conventions
This guide uses specific conventions for presenting information that may require
your attention. Refer to the following safety alert conventions.
Safety Alerts for Chemicals
Fluidigm follows the United Nations Globally Harmonized System (GHS) for
communicating chemical hazard information. GHS provides a common means of
classifying chemical hazards and a standardized approach to chemical label
elements and safety data sheets (SDSs). Key elements include:
•Pictograms that consist of a symbol on a white background within a red diamond-
shaped frame. Refer to the individual SDS for the applicable pictograms and
warnings pertaining to the chemicals being used.
•Signal words that alert the user to a potential hazard and indicate the severity level.
The signal words used for chemical hazards under GHS:
DANGER Indicates more severe hazards.
WARNING Indicates less severe hazards.
Safety Alerts for Instruments
For hazards associated with instruments, this guide uses the following indicators:
•Pictograms that consist of a symbol on a white background within a black triangle-
shaped frame.

Genotyping with Juno Getting Started Guide
About This Guide
Safety Data Sheets
6
•Signal words that alert the user to a potential hazard and indicate the severity level.
The signal words used for instrument hazards:
DANGER Indicates an imminent hazard that will result in severe injury or death if not
avoided.
WARNING Indicates a potentially hazardous situation that could result in serious
injury or death.
CAUTION Indicates a potentially hazardous situation that could result in minor or
moderate personal injury.
IMPORTANT Indicates information necessary for proper use of products or
successful outcome of experiments.
Safety Data Sheets
Read and understand the SDSs before handling chemicals. To obtain SDSs for
chemicals ordered from Fluidigm Corporation, either alone or as part of this system,
go to fluidigm.com/sds and search for the SDS using either the product name or the
part number.
Some chemicals referred to in this user guide may not have been provided with your
system. Obtain the SDSs for chemicals provided by other manufacturers from those
manufacturers.
Genotyping with Juno Getting Started Guide 7
Getting Started
Workflow
Best Practices
•Use good laboratory practices to minimize contamination of samples. Use a new
pipette tip for every new sample. Whenever possible, separate pre- and post-
PCR activities. Dedicate laboratory materials to designated areas.
•Unless otherwise specified, thaw reagents at room temperature, then use them
at room temperature. Store reagents at their specified storage temperatures.
(See “Required Kit Contents” on page 8.)
•Vortex reagents for 20 seconds, and then centrifuge reagents for 2 seconds
before use.
Related Documentation
Go to fluidigm.com/documents
Reagent Handling Automated Steps Estimated Times
1Prepare preamplification and
genotyping assay and sample mixes
30–60 minutes
2Pipet preamplification, genotyping
mixes, and control line fluid into the IFC
10–20 minutes
3Run a script to preamplify and
genotype the DNA
2.5 hours (TaqMan
protocol); 3.5 hours
(SNP Type protocol)
4Perform genotyping analysis on
EP1 or Biomark systems
5–10 minutes

Genotyping with Juno Getting Started Guide
8
Chapter 1: Product Information
Required Kit Contents
The kits include the reagents required for preparing 10 IFCs to use on the Juno
system. For suggested kits, see “Suggested Kits” on page 28.
IMPORTANT
• Do not pipet reagents from the TaqMan and SNPType assay kits into the same IFC.
Use a different IFC for each kit. Do not mix reagents from different kits.
• Unless otherwise specified, thaw reagents at room temperature, then use them at
room temperature. Store reagents at their specified storage temperatures. Vortex
reagents for 20 seconds, then centrifuge reagents for 2 seconds before use.
TaqMan Assay Kit
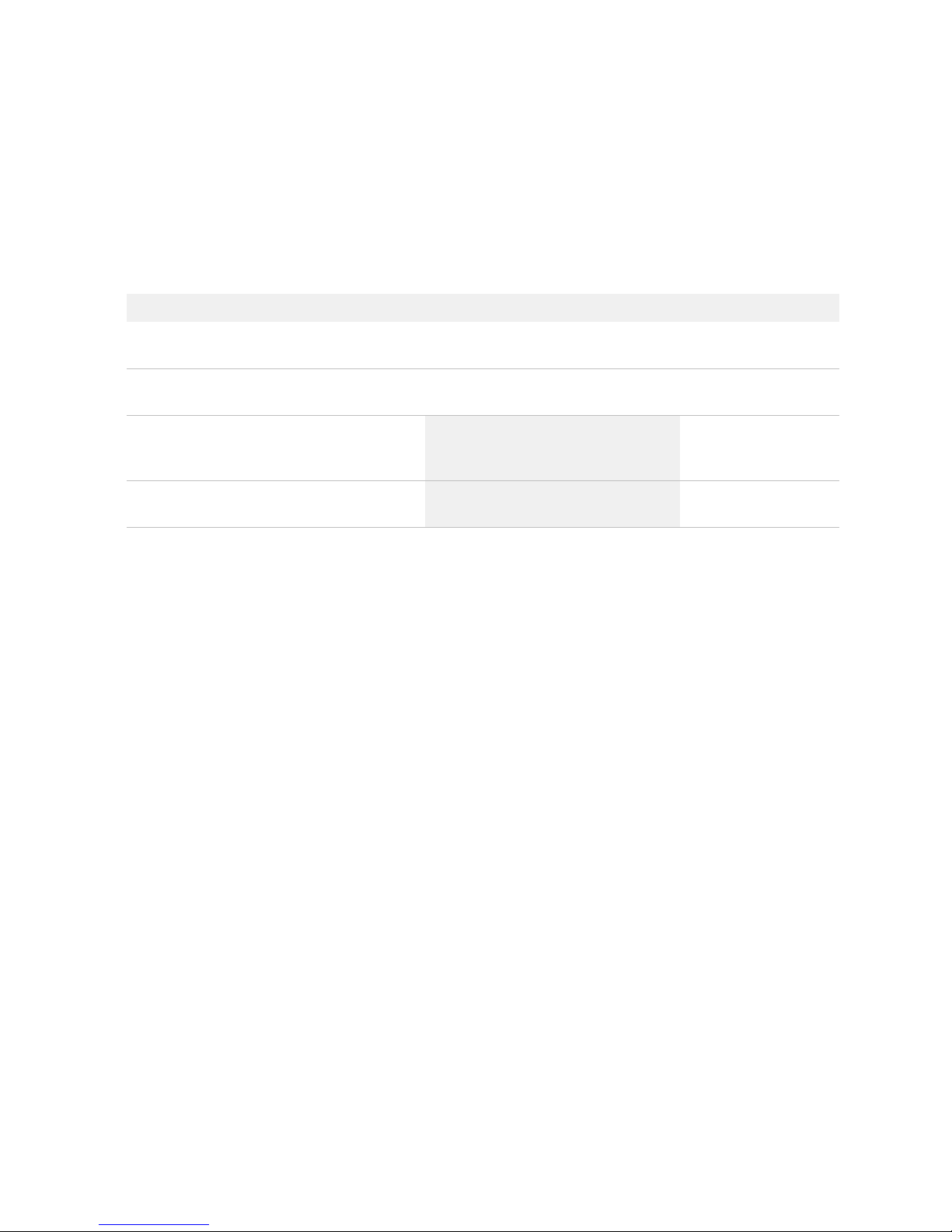
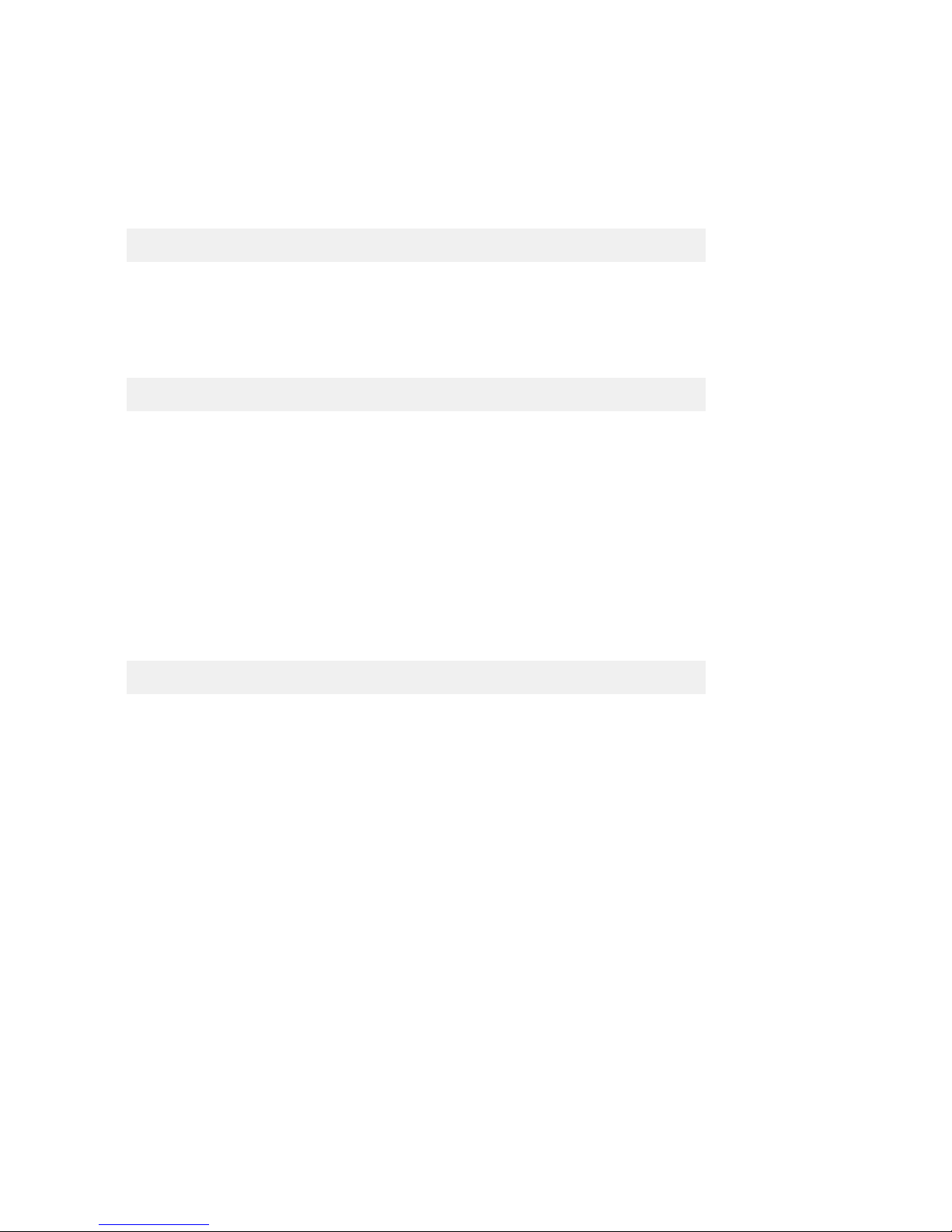
Figure 1. Juno™ Genotyping Kit for 10 IFCs (PN 100-8362).
Box Component Cap Color Quantity Volume
per Tube (mL)
Storage
Juno Genotyping Kit
for 10 IFCs
(PN 100-8362)
Juno GT Preamp
Master Mix
Light
purple
1 tube 1.35
–20 ºC
Dilution Reagent Natural 2 tubes 1.7
Probe GT Master Mix Gold 2 tubes 1.6
Juno GT Flux Fluid Purple 1 tube 0.9
Juno 96.96
Genotyping IFC—
10 IFCs
— 10 IFCs —
Room
temperature
Juno 96.96 GT
Control Line Fluid
— 2 boxes;
20 syringes/
box
—
Probe GT Master Mix
Juno GT Flux Fluid Juno GT Preamp Master Mix
Dilution Reagent
Genotyping with Juno Getting Started Guide
Chapter 1: Product Information
Required Kit Contents
9
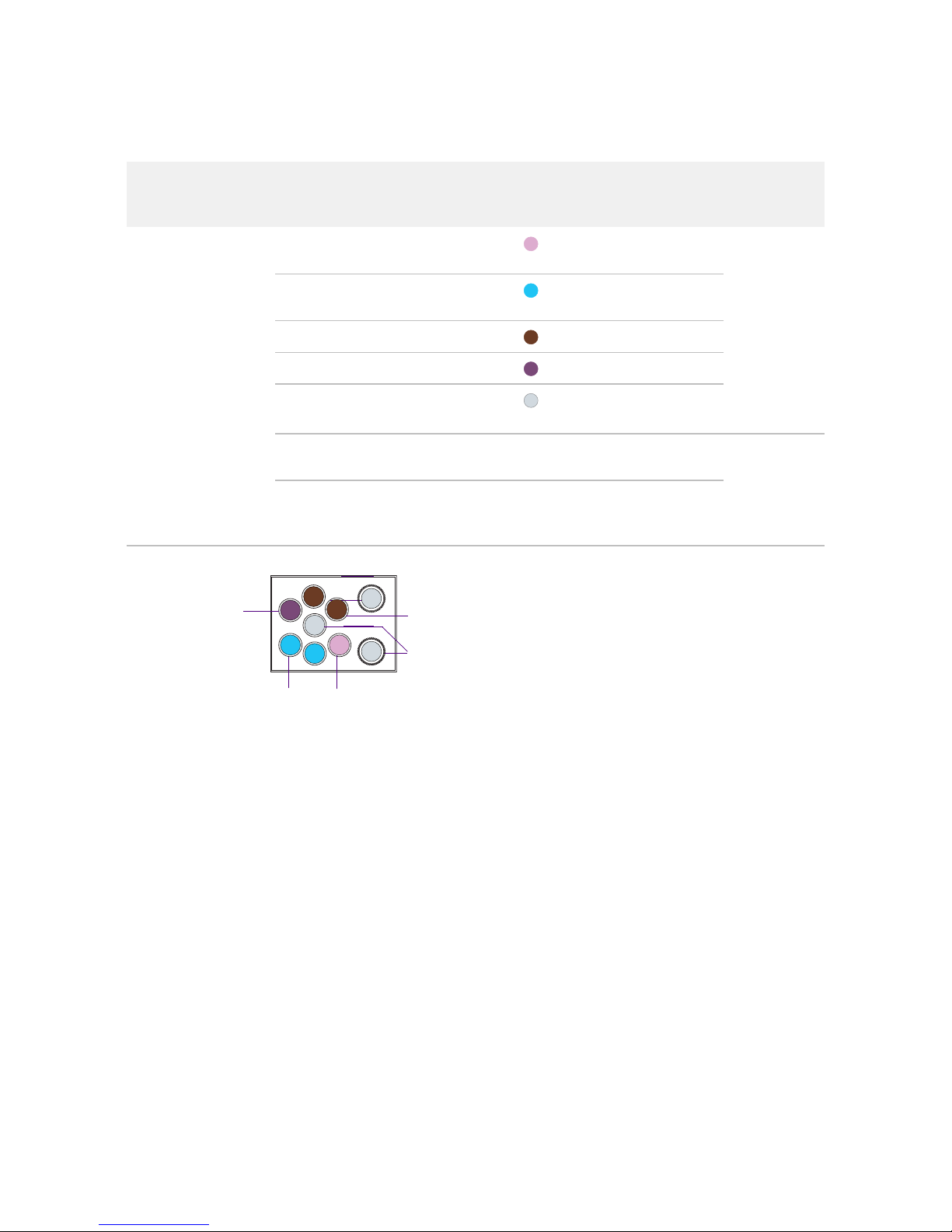
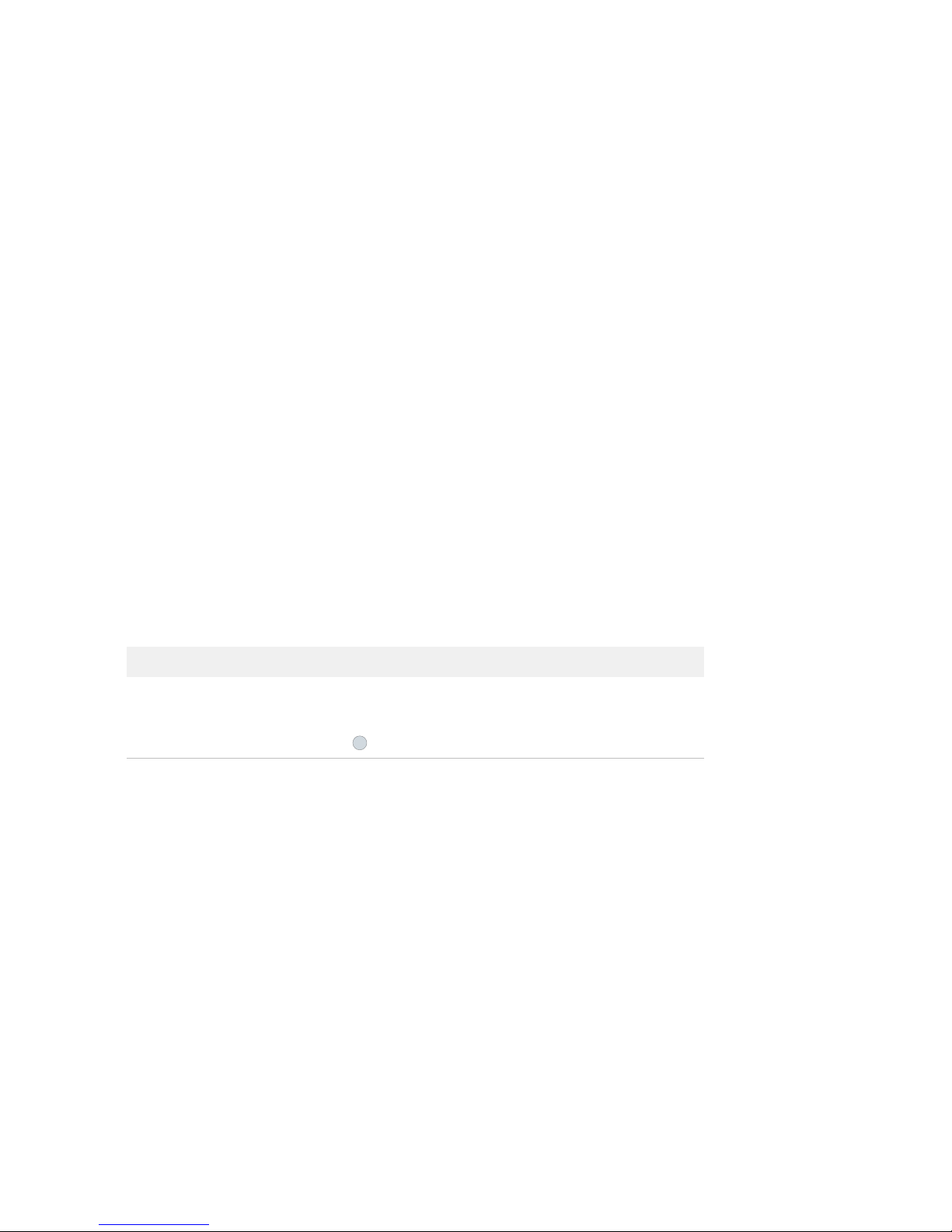
SNP Type Assay Kit
Figure 2. Juno™ SNP Type Genotyping Kit for 10 IFCs (PN 100-8364).
Box Component Cap Color Quantity Volume
per Tube
or Bottle
Storage
Juno SNP Type
Genotyping Kit for
10 IFCs
(PN 100-8364)
Juno GT Preamp Master
Mix
Light
purple
1 tube 1.35 mL
–20 ºC
Juno SNP Type GT
Master Mix
Light
blue
2 tubes 1.6 mL
60X SNP Type Reagent Amber 2 tubes 70 μL
Juno GT Flux Fluid Purple 1 tube 1.0 mL
Dilution Reagent Natural •2 bottles
•1 tube
•3.7 mL
•1.7 mL
Juno 96.96 Genotyping
IFC—10 IFCs
— 10 IFCs —
Room
temperature
Juno 96.96 GT Control
Line Fluid
— 2 boxes;
20 syringes/
box
—
60X SNP Type Reagent
Juno GT Flux Fluid
Juno GT Preamp Master Mix
Dilution Reagent
Juno SNP Type GT Master Mix
Genotyping with Juno Getting Started Guide
Chapter 1: Product Information
Required Reagents
10
Required Reagents
TaqMan Assays
SNP Type Assays
Suggested Reagents
Product Name Company Part Number
20X, 40X, or 80X TaqMan®genotyping
assays
Thermo Fisher
Scientific
—
Product Name Company Part Number
SNP Type assays specific target
amplification Primers (100 μM STA)
Fluidigm —
SNP Type assays ASP1/ASP2
(100 μM each)
Fluidigm —
SNP Type assays LSP
(100 μM each)
Fluidigm —
Product Name Company Part Number
UltraPure™DNase/RNase-Free Distilled
Water
Thermo Fisher
Scientific
10977-015

Genotyping with Juno Getting Started Guide
Chapter 1: Product Information
Required Consumables
11
Required Consumables
Required Equipment
Suggested Equipment
Product Name Company Part Number
Juno 96.96 Genotyping IFC:
•Juno 96.96 Genotyping IFC
•Juno 96.96 Genotyping IFC, 10 Pack
Fluidigm 100-6499
100-8365
Disposable microcentrifuge tubes,
polypropylene, 1.5 mL
Major laboratory
supplier (MLS)*
* Recommended: VWR®Slick Disposable Microcentrifuge Tubes, Polypropylene, 1.5 mL
(VWR PN 20170-666)
—
96-well PCR plates MLS†
† Recommended: TempPlate®semi-skirted 96-well PCR plates (USA Scientific
PN 1402-9700)
—
MicroAmp® Clear Adhesive Film Thermo Fisher
Scientific
4306311
Product Name Company Part Number
Juno system, including system software version v3.1 or
later, instrument, software, MX Interface Plate, Interface
Plate Loading Fixture, Cleaning Plate, and Barrier Tape
Applicator and Adapter
Fluidigm 101-6455
For Juno 96.96 Genotyping IFC: SX Interface Plate Fluidigm 100-6368
Vortexer MLS —
Pipettes (P2, P20, P200, P1000) and appropriate
low-retention tips
MLS —
8-channel pipettes and appropriate low-retention tips MLS —
Microcentrifuge MLS —
Product Name Company Part Number
Two biocontainment hoods (DNA hood and DNA-free hood)
to prevent DNA contamination of lab and samples
MLS —

Genotyping with Juno Getting Started Guide
Chapter 1: Product Information
Required Software
12
Required Software
•Fluidigm Data Collection software v4.2 or later
•Fluidigm SNP Genotyping Analysis software v4.2 or later
IFC Type and Related Scripts
Barcode (prefix) Scripts Description
180x Juno 96.96 Fast Preamplification and genotyping of samples by
TaqMan assays (180x)
180x Juno 96.96 Preamplification and genotyping of samples by SNP
Type assay (180x).
Genotyping with Juno Getting Started Guide 13
Chapter 2: Genotyping with the
Juno 96.96 Genotyping IFC Using
TaqMan Assays
Prepare Assay and Sample Mixes
Prepare the Primer Pool for Preamplification
1If necessary, adjust the concentration of TaqMan genotyping assays with DNase-free
water to 18 μM (20X).
2In a new, labeled 1.5-mL microcentrifuge tube, combine 2 μL of each 20X TaqMan
genotyping assay up to a total of 96 assays. The total volume of assays is 2Yin
Table 1, where Yis the number of assays used. Each assay is at a final concentration
of 0.2X in the primer pool.
3Add Dilution Reagent to the 20X TaqMan assays:
Table 1. Prepare the primer pool for preamplification
The final concentration of each primer in the preamplification reaction is 45 nM.
NOTE The volume can be adjusted proportionally based on the number of samples
to be amplified.
Component Volume (μL) Final Concentration
20X TaqMan genotyping assays,
18 μM*
* See step 1.
2Y (up to 96 assays) 180 nM (0.2X)
Dilution Reagent 200 – 2Y—
Total 200.0 —
Genotyping with Juno Getting Started Guide
Chapter 2: Genotyping with the Juno 96.96 Genotyping IFC Using TaqMan Assays
Prepare Assay and Sample Mixes
14
Prepare 2X TaqMan Assays for Genotyping
1If necessary, adjust the concentration of TaqMan genotyping assays with DNase-free
water to 18 μM (20X).
2In a new 96-well plate, dilute the 20X TaqMan genotyping assays in Dilution
Reagent or DNase-free water to a final concentration of 2X for each assay:
Prepare the Assay Mix
1Label a new 96-well plate, “TAQMAN ASSAY PLATE.” In a DNA-free hood, pipet
2.5 μL of Probe GT Master Mix into each well. (See Table 2.)
2In a DNA-free hood, pipet 2.5 μL of 2X TaqMan assays into a well of the TaqMan
assay plate for each assay. (See “Prepare 2X TaqMan Assays for Genotyping”.)
3In unused assay inlets, combine 2.5 μL of Probe GT Master Mix with 2.5 μL DNase-
free water.
4Seal the plate with MicroAmp Clear Adhesive Film, vortex it for 5 seconds, then
centrifuge it at 1,000 x gfor 1 minute.
Table 2. Assay mix
Obtain the Minimum Required Genomic DNA
For high-quality human samples, the minimum DNA required is 2.5 ng/μL in 2.75 μL.
Larger genomes require higher concentrations of genomic DNA.
Component Volume (μL) Final Concentration
20X TaqMan genotyping assays 1.0 2X
Dilution Reagent or
DNase-free water
9.0 —
Total 10.0 —
Component Volume (μL)
Probe GT Master Mix 2.5
2X TaqMan assays*
* See “Prepare 2X TaqMan Assays for Genotyping”.
2.5
Total 5.0
Genotyping with Juno Getting Started Guide
Chapter 2: Genotyping with the Juno 96.96 Genotyping IFC Using TaqMan Assays
Prepare Assay and Sample Mixes
15
Prepare the Sample Mix
1In a DNA-free hood, in a new 1.5-mL microcentrifuge tube labeled “Sample Pre-Mix,”
combine the Juno GT Preamp Master Mix and the primer pool for preamplification to
prepare the sample pre-mix. (See Table 3.)
2Label a new 96-well plate “SAMPLE PLATE.” Pipet 2.25 μL of the sample pre-mix
into each well of the plate. Skip wells that are for no template controls. Do not add
sample pre-mix to no template control wells.
IMPORTANT Prepare at least one no template control.
3In a DNA sample hood, pipet 2.75 μL of genomic DNA into the appropriate wells of
the sample plate.
4In a DNA sample hood, pipet 5.00 μL of Dilution Reagent into each no template
control well.
5Seal the plate with MicroAmp Clear Adhesive Film, vortex it for 5 seconds, then
centrifuge it at 1,000 x gfor 1 minute.
Table 3. Sample mix
IMPORTANT Do not go past the first stop on the pipette. Doing so may introduce air
bubbles into the inlets. To avoid bubbles, pipet 4.0 μL into each inlet from the 5.0 μL
overage volume.
Component Volume per
Inlet (μL)
Volume per
Inlet with
Overage
(μL)
Sample Mix
for IFC with
Overage*
(μL)
* 120 reactions for ease of pipetting
SAMPLE PRE-MIX
Juno GT Preamp Master Mix 0.8 1.00 120.0
Primer pool for preamplification†
† See “Prepare the Primer Pool for Preamplification” on page 13.
1.0 1.25 150.0
Genomic DNA 2.2 2.75 —
Total 4.0 5.0 270.0

Genotyping with Juno Getting Started Guide
Chapter 2: Genotyping with the Juno 96.96 Genotyping IFC Using TaqMan Assays
Load and Run the IFC on Juno
16
Load and Run the IFC on Juno
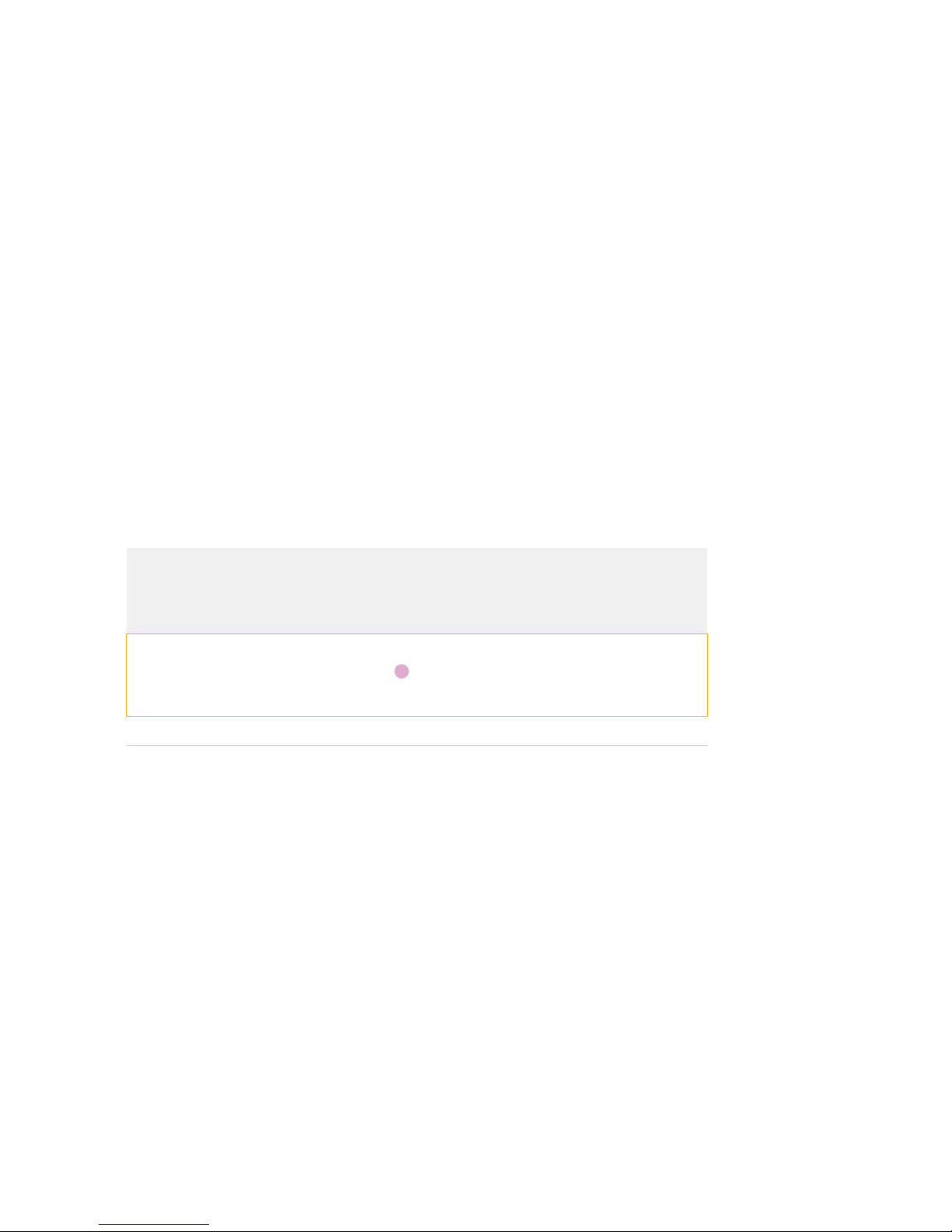
1Review the loading map, which is affixed to the bottom of every new Juno 96.96
Genotyping IFC. The loading map is a general guide to show you how to pipet
samples, assays, flux fluid, and control line fluid:
A1

Genotyping with Juno Getting Started Guide
Chapter 2: Genotyping with the Juno 96.96 Genotyping IFC Using TaqMan Assays
Load and Run the IFC on Juno
17
2Review the pipetting map, which provides specific instructions for pipetting reagents
in the IFC. Pipet reagents from the TaqMan assay plate and the sample plate to the
IFC. On the pipetting map, each inlet is labeled with the plate well location of the
sample or assay to be pipetted into that inlet:
Figure 1. Pipetting map for the Juno 96.96 Genotyping IFC
3Ensure that the notched corner of the IFC (“A1”) is at the top left.
4Load an entire syringe of Juno 96.96 GT Control Line Fluid in Acc1 and a second
syringe in Acc2. (See pink squares on the pipetting map.) To ensure correct
accumulator volume, only use syringes containing Juno 96.96 GT Control Line Fluid.
Key
Load 1 Load 2
Juno 96.96 GT Control
Line Fluid Assay mix, 4.0 μL
Juno GT Flux Fluid,
15 μLSample mix, 4.0 μL
Juno 96.96 GT Control
Line Fluid
—Empty
vv
Acc 1
Acc 2
A1
A01 A02 A03 A04 A05 A06
A07 A08 A09 A10 A11 A12
B01 B02 B03 B04 B05 B06
B07 B08 B09 B10 B11 B12
C01 C02 C03 C04 C05 C06
C07 C08 C09 C10 C11 C12
D01 D02 D03 D04 D05 D06
D07 D08 D09 D10 D11 D12
E01 E02 E03 E04 E05 E06
E07 E08 E09 E10 E11 E12
F01 F02 F03 F04 F05 F06
F07 F08 F09 F10 F11 F12
G01 G02 G03 G04 G05 G06
G07 G08 G09 G10 G11 G12
H01 H02 H03 H04 H05 H06
H07 H08 H09 H10 H11 H12
A01 A02 A03 A04 A05 A06
A07 A08 A09 A10 A11 A12
B01 B02 B03 B04 B05 B06
B07 B08 B09 B10 B11 B12
C01 C02 C03 C04 C05 C06
C07 C08 C09 C10 C11 C12
D01 D02 D03 D04 D05 D06
D07 D08 D09 D10 D11 D12
E01 E02 E03 E04 E05 E06
E07 E08 E09 E10 E11 E12
F01 F02 F03 F04 F05 F06
F07 F08 F09 F10 F11 F12
G01 G02 G03 G04 G05 G06
G07 G08 G09 G10 G11 G12
H01 H02 H03 H04 H05 H06
H07 H08 H09 H10 H11 H12
Genotyping with Juno Getting Started Guide
Chapter 2: Genotyping with the Juno 96.96 Genotyping IFC Using TaqMan Assays
Load and Run the IFC on Juno
18
5Load an entire syringe of Juno 96.96 GT Control Line Fluid into a reservoir and a
second syringe into the second reservoir. (See long pink rectangles on the right side
of the pipetting map.)
IMPORTANT Carefully dispense control line fluid into the reservoirs. If control line
fluid comes into contact with the sample inlets, use a new IFC.
6Pipet 15 μL of Juno GT Flux Fluid into each of the six ports. (See purple circles on the
pipetting map.)
7Unseal the TaqMan assay plate and pipet 4.0 μL of each assay mix into an assay
inlet. (See black circles on the pipetting map and “Prepare the Assay Mix” on
page 14.)
8Unseal the sample plate and pipet 4.0 μL of each sample mix into a sample inlet.
(See green circles on the pipetting map and “Prepare the Sample Mix” on page 15.)
IMPORTANT Do not go past the first stop on the pipette. Doing so may introduce air
bubbles into the inlets. Pipet 4.0 μL from the 5.0 μL overage volume to ensure that
no air bubbles enter the inlet.
9Pull the sticker front tab down and away from the IFC to gently peel off the loading
map. Do not invert the IFC.
10 If necessary, remove any bubbles from an IFC inlet by removing the contents by
pipette and then carefully re-pipetting the contents into the inlet.
11 Ensure that the SX interface plate (silver label) is installed in the instrument. [See the
Juno System User Guide (PN 100-7070).]
12 Place the IFC into the Juno instrument, then start the run <60 minutes after pipetting
the reagents into the IFC.
13 On the Juno Scripts screen, tap the Probe GT tab, Juno 96.96 Fast, then Run. It
takes ~2.7 hours to complete.
The script contains these thermal cycling protocols:
Cycles Temperature Time
Hot start 95 ºC 2 min
14 95 ºC 15 sec
60 ºC 4 min
Cycles Temperature Time
Hot start 95 ºC 2 min
45 95 ºC 2 sec
60 ºC 20 sec

Genotyping with Juno Getting Started Guide
Chapter 2: Genotyping with the Juno 96.96 Genotyping IFC Using TaqMan Assays
Perform Genotyping Analysis on the Samples
19
14 After the run is finished, tap EJECT to eject the IFC.
IMPORTANT After a run, perform an end-point read of the IFC in ≤1 hour. Do not
leave the IFC overnight in the instrument. Doing so will adversely affect the reaction.
Perform Genotyping Analysis on the
Samples
Refer to the appropriate document:
•SNP Genotyping User Guide (PN 68000098)
•Biomark HD Data Collection User Guide (PN 100-2451)
•Biomark/EP1 Data Collection User Guide (PN 68000127).
Genotyping with Juno Getting Started Guide
20
Chapter 3: Genotyping with the Juno
96.96 Genotyping IFC Using SNP Type
Assays
Prepare Assay and Sample Mixes
Prepare the 200 nM Primer Pool for Preamplification
1In a new 1.5-mL microcentrifuge tube, combine 2 μL of 100 μM SNP Type assays
specific target amplification primers (100 μM STA) up to a total of 96 assays. The total
volume is Yin Table 4.
2In the same microcentrifuge tube, combine 2 μL of 100 μM SNP Type assays locus-
specific primers (100 μM LSP) up to a total of 96 assays. The total volume is Zin
Table 4.
3Add Dilution Reagent to the SNP Type assays:
Table 4. Pool SNP Type assays
NOTE
• Volume can be adjusted proportionally based on the number of
samples to be amplified.
• You can store the pooled SNP Type STA assays at –20 °C for 1 year or ≤10
freeze-thaw cycles, whichever is shorter.
Component Volume (μL) Final Concentration
(nM*)
* The final concentration of each primer in the preamplification reaction is 50 nM.
SNP Type assays specific target amplification
primers (100 μM STA)
Y(up to 96 assays) 200.0
SNP Type assays locus-specific primers
(100 μM LSP)
Z(up to 96 assays) 200.0
Dilution Reagent 1,000 – (Y+ Z)—
Total 1,000.0 —
Other manuals for Juno
1
Table of contents
Other Fluidigm Laboratory Equipment manuals
Popular Laboratory Equipment manuals by other brands

StarTech.com
StarTech.com POEINJ1G90W quick start guide

LevelOne
LevelOne IGP-0102 user manual

Leica
Leica AutoStainerXL Instructions for use

Getinge
Getinge GE 6610 AC-1 Service manual

PARCUS MEDICAL
PARCUS MEDICAL Knotless PEEK CF Important product information
Renfert
Renfert Easyclean TEC Translation of the original manual