1
Introduction /
English
/ 1 Introduction
/ 1.1 Denitions
•Device GlucoMen Day PENCAP
•User Insulin pen user, Patient or Operator
•App GlucoLog RapidCalc app
/ 1.2 General description
GlucoMen Day PENCAP helps you track your insulin injections. It can stre-
amline your diabetes management by automatically logging injections into
a diabetes management app. You can also easily see the time since last
injection on the display, helping you to stay on top of your injection routine.
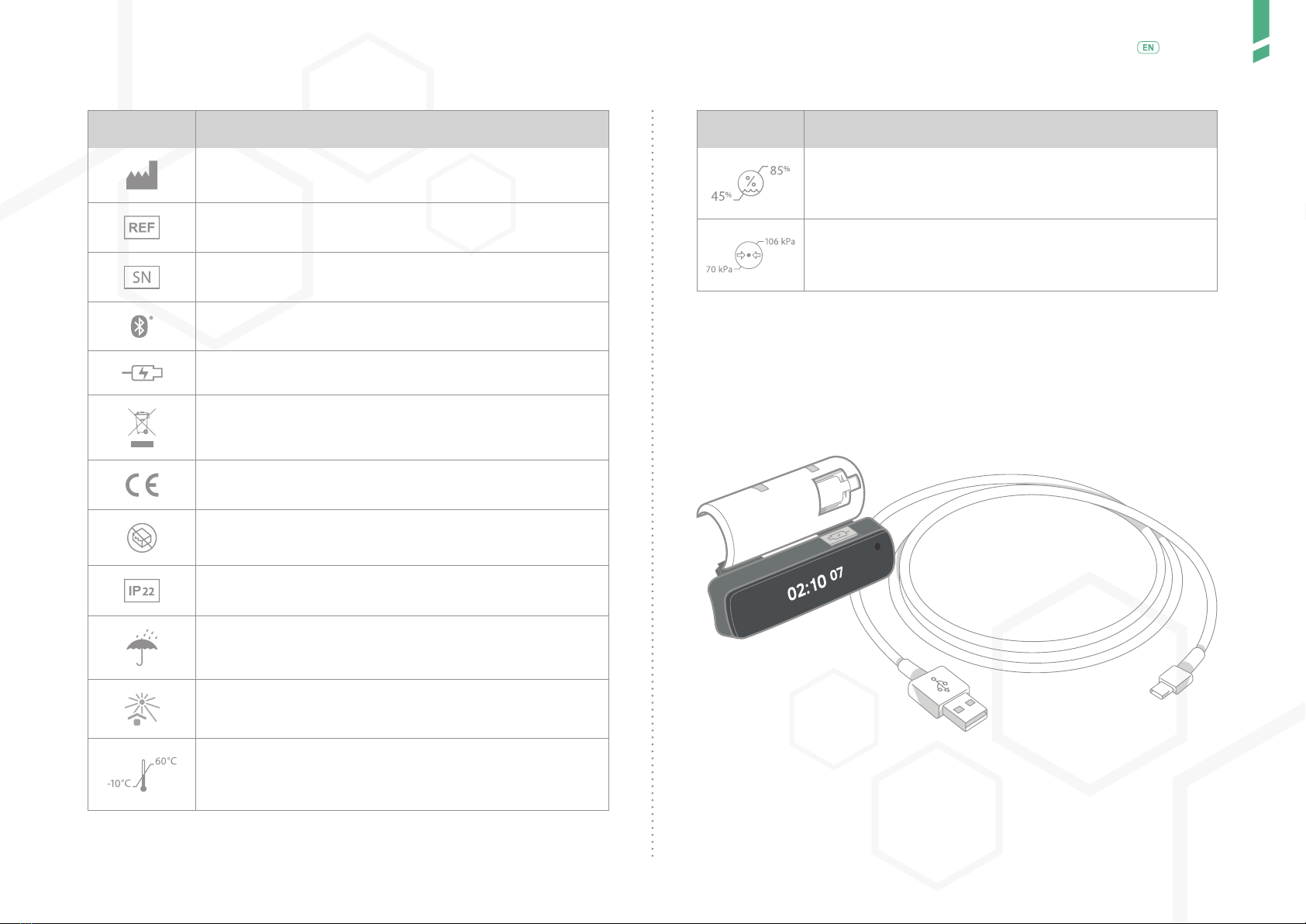
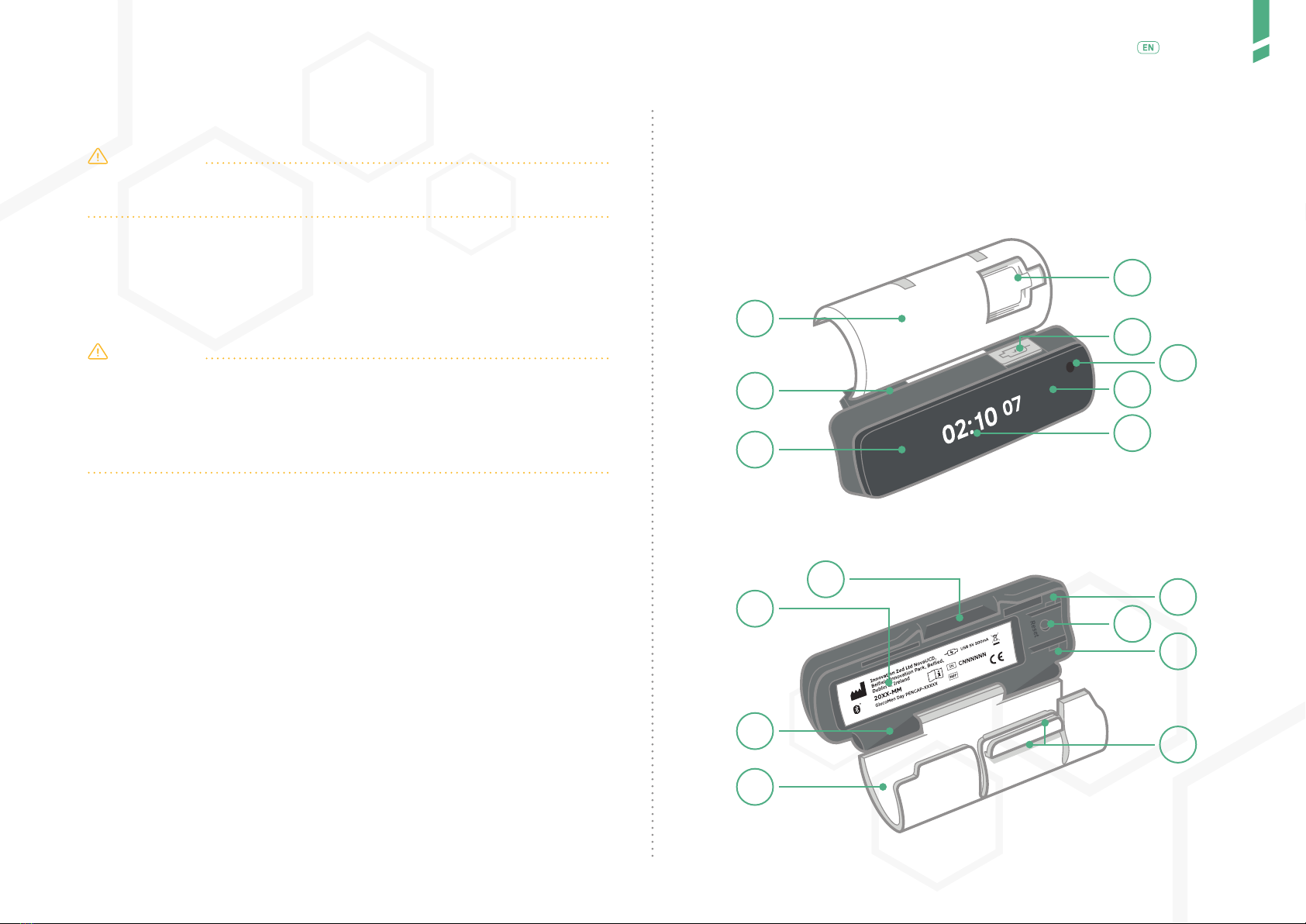
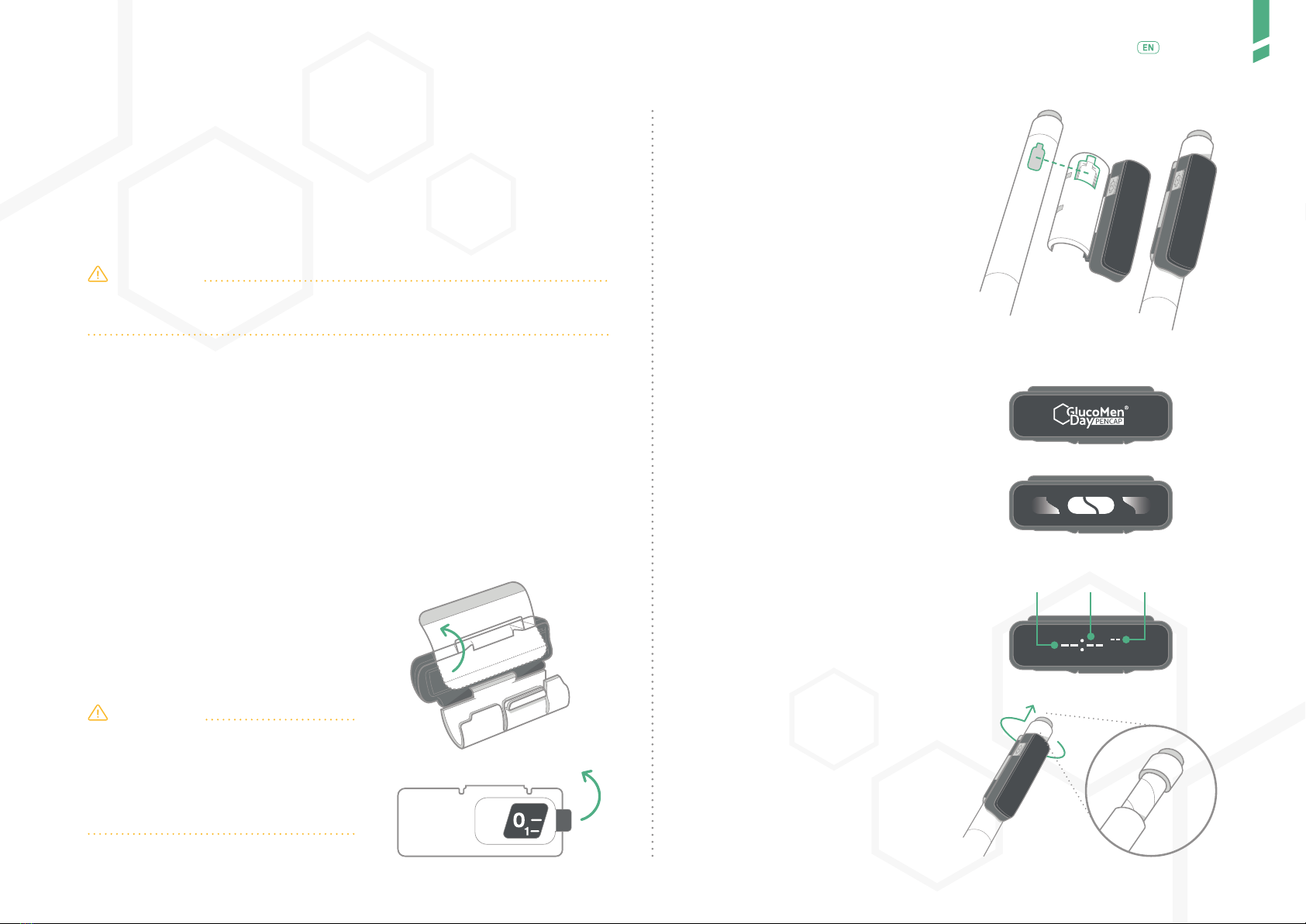
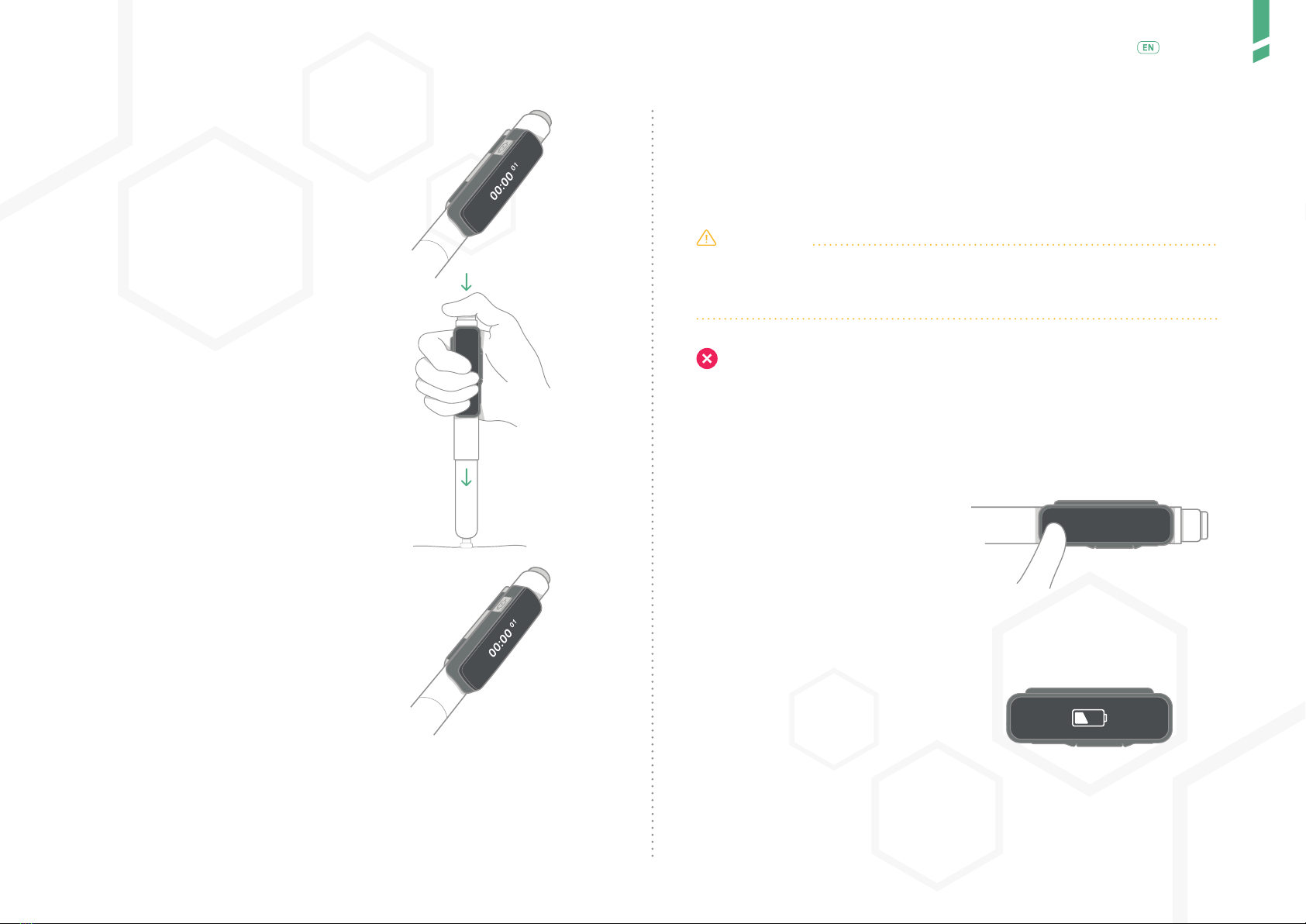
GlucoMen Day PENCAP is clipped-on to your insulin injection pen. Once moun-
ted on the pen, it automatically detects when you dial-out a dose and perform
an injection. The Device starts counting up the time after you have injected.
To use the GlucoMen Day PENCAP you need to install the GlucoLog RapidCalc
app (available for both Android on the Play Store, and iOS on the Apple
Store) on your smartphone.
/ 1.3 Intended use
GlucoMen Day PENCAP is a long-term secondary aid in diabetes manage-
ment, by providing information about the timing of injections of insulin.
The timer on the GlucoMen Day PENCAP must NOT be used as the
only indicator to decide when to inject insulin. You must rely on your own
memory and common sense, your blood glucose level and other informa-
tion you have to decide when to perform an insulin injection.
The Device is designed to be used in common domestic environments
such as your home, oce or school.
/ 1.4 Intended Users
GlucoMen Day PENCAP is intended for insulin pen users who are familiar
with the use of these pens.
GlucoMen Day PENCAP should NOT be used as the sole or main method
of managing the timing of injections or by users who are unable to ma-
nage their treatment without the use of the Device.
CAUTION
The User must be able to safely use all functions of the Device.
/ 1.5 Normal use
GlucoMen Day PENCAP is mounted on the insulin pen by the user. The
Device is on the pen when injecting. The time since previous injection is
displayed on the display screen. Using Bluetooth® technology, data from
the Device can be transferred to a connected app (GlucoLog RapidCalc app).
Once mounted, the Device need not be removed from reusable pens.
When used with disposable pens, once the insulin runs out, the Device
is removed and mounted on a new disposable pen. GlucoMen Day PEN-
CAP has a lithium-ion battery that is rechargeable when a low battery is
indicated.
/ 1.6 Symbols
The following table describes the meaning of the symbols used in the
User Manual, the Packaging and the Label on the bottom of the Device.
Symbol Meaning
This action is prohibited. User should read very
carefully.
Important information, warning or precaution. User
should read carefully.
Read User Manual before use.