Health & Life HL858DC User manual

I
In
ns
st
tr
ru
uc
ct
ti
io
on
n
M
Ma
an
nu
ua
al
l
Automatic
Upper Arm Blood Pressure Monitor
Model No. HL858DC
Medical Disclaimer……………………………………………………………………………03
Intended Use………………………………………………………………………………..…03
About Blood Pressure………………………………………………………………………04
Measurement Method……………………………………………………………………….06
Accuracy…………………….……………………………………………………………………. 06
Precautions…………………….…………………………………………………………………07
Device Overview……………………………………………………………………………….09
Symbol Definitions………………………………………………………………………….11
Features……………………………………………………………………………………………12
Installing Batteries………………………………………………………………………….16
Using the AC Adapter………………………………………………………………………17
Applying the Cuff…………………………………………………………………………….18
Measurement Procedure………….…………………………………………………….19
Memory Function…………………………………………………………………………….21
Bluetooth Communication………….……………………………………………………22
Storage and Maintenance……………………………………………………………….23
Troubleshooting……………………………………………………………………………….24
Warranty and Recalibration …………………………………………………………….25
Specifications………………………………………………………………………………….26
Note………………………………………………………………………………………….…….28
Appendix…………………………………………………………………………………….…….30
Blood Pressure Diary……………….……………………………………………….…….32
Table of Contents
1 2

This manual and product are not meant as a substitute for advice
provided by your doctor.
You are not to use the information contained herein, or this product
for diagnosing or treating a health problem or prescribing any
medication. If you have or suspect that you have a medical problem,
promptly consult your healthcare provider.
This device uses the oscillometric method to automatically measure
systolic and diastolic blood pressure as well as heart rate.
The measurement position is at human being’s arm.
All values can be read out in one LCD panel.
The device is designed for home use and recommended for use by
adults aged 18 years and older with upper arm circumference ranging
from 9 ~13” (approx. 23 ~ 33 cm).
1. What is blood pressure?
Blood pressure is the measurement of the force of blood pushing
against the walls of the arteries. Arterial blood pressure is
constantly fluctuating during the course of the cardiac cycle. The
highest pressure in the cycle is called the systolic blood pressure,
and represents the pressure in theartery when the heart is beating.
The lowest pressure is the diastolic blood pressure, and represents
the pressure in the artery when the heart is at rest. Both the
systolic and the diastolic pressure are necessary for a physician to
evaluate the status of a patient's blood pressure.
Many factors such as physical activity, anxiety or the time of day,
can influence your blood pressure. Blood pressure is typically low
in the mornings and increases from the afternoon to the evening.
It is on average lower in the summer and higher in the winter.
2. Why is it useful to measure blood pressure
at home?
Having one's blood pressure measured by a doctor in a hospital or
a clinic, is often associated with a phenomenon called “White Coat
Hypertension” where the patient becomes nervous or anxious,
thus raising his blood pressure. There are also numerous other
factors that might cause your blood pressure to be raised at a
specific time of day. This is why medical physicians recommend
home monitoring as it is important to get readings of blood
pressure during different times of the day to really get an idea of
your real blood pressure.
Medical physicians generally recommend the “Rule of 3”, where
you are encouraged to take your blood pressure three times in a
row (at 3 ~ 5 minute interval), three times a day for three days.
After three days you can average all the results and this will give
you an accurate idea of what your blood pressure really is.
Intended Use
About Blood Pressure
Medical Disclaimer
3 4
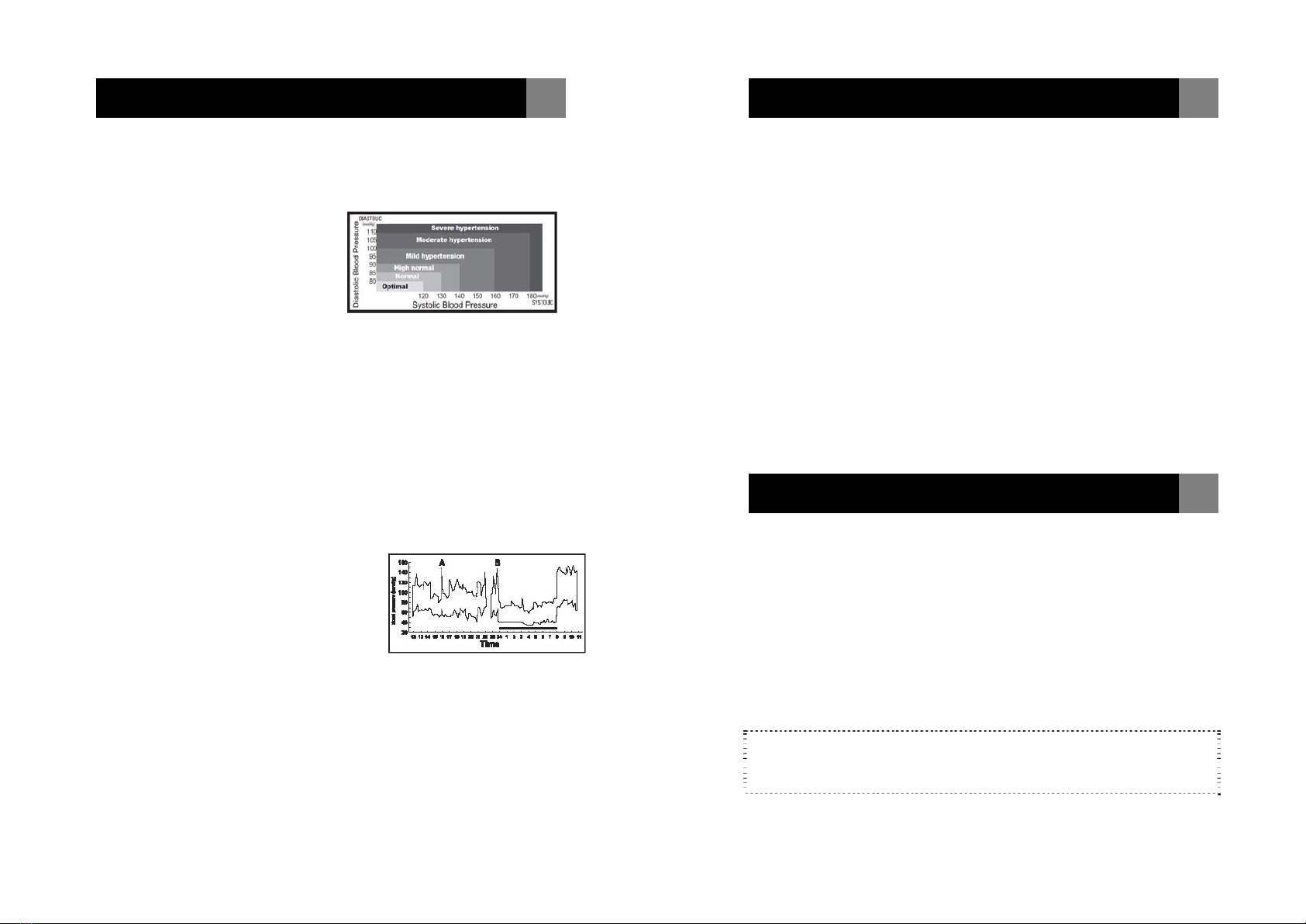
A.
Standards for assessment of high or low blood pressure
without regard to age, have been established by the WHO,
and classifications adapted from JNC7:
WHO
: World
Health
Organization
JNC 7: The Seventh Report of
the Joint National Committee
on Prevention, Detection,
Evaluation, and Treatment of
High Blood Pressure. NIH
Publication No.04-5230 August 2004
However the above chart is not exact for classification of blood
pressure and it's intended to be used as a guide in
understanding non-invasive blood pressure measurements.
Please consult with your physician for proper diagnosis.
B.
Variations in blood pressure:
Individual blood pressures vary greatly both on a daily and a
seasonal basis. These variations are even more pronounced in
hyper tense patients. Normally the blood pressure rises while at
work and is at its lowest during sleeping period.
(hyper tense: means a person who has high blood
pressure symptom.)
The graph below illustrated the
variations in blood pressure over a
whole day with measurement taken
every five minutes.
The thick line represents sleep. The
rise in blood pressure at 4 PM (A in
the graph) and 12 PM (B in the graph) correspond to an attack
of pain.
HL858DC Automatic Upper Arm Blood Pressure Monitor measures
blood pressure and heart rate by oscillometric method, meaning the
fluctuations in pressure are measured. Once the cuff is wrapped
around your upper arm, just turn on the monitor and inflation
automatically starts. The inflation of the cuff creates pressure around
the arteries inside upper arm.
Within the cuff is a gauge which senses the fluctuations (oscillations)
in pressure. The fluctuation measured represents the degree of
intensity that your arteries contracting with each heart beat, and also
a result of the pressure that the cuff has placed on the upper arm. The
monitor measures these contractions and converts the information to
a digital value. This is the result displayed on the monitor screen.
Once the measurement is complete, the cuff will automatically
deflate.
HL858DC Automatic Upper Arm Blood Pressure Monitor has been
clinically tested against a scientific device called sphygmomanometer,
considered the gold standard in blood pressure measurement.
All HL858DC Automatic Upper Arm Blood Pressure Monitors have
performed equivalent to measurements taken with this scientific
device and are within the accuracy limits prescribed by the American
National Standard for Electronic or Automated
Sphygmomanometers.
*We suggest our users have their blood pressure monitor
checked every 2 years. This operation should only be
performed by Manufacturer or by authorized representatives.
Accuracy
Measurement Method
About Blood Pressure
5 6

Do not take a measurement in a low (less than 41 ℉/5 ℃) and
high (more than 104 ℉/40 ℃) temperature, nor in a place outside
humidity ranges (15 % ~ 93 % R.H.), or you may get inaccurate
readings.
Wait 30 ~ 45 minutes before measurement if you’ve just
consumed caffeinated beverages or smoked cigarettes.
Rest at least 5 ~ 10 minutes before taking a measurement.
To allow your blood vessels to return to the condition prior to
taking the measurement, please wait at least 3 ~ 5 minutes in
between measurements. You may need to adjust the wait time
according to your personal physiological situation.
We recommend you using the same arm (preferably the left arm)
and measuring around the same time each day.
Sit down comfortably and place your elbow on the table with your
feet flat on the floor. Please do not cross your legs during
measurements.
Keep the cuff at heart level. Relax your hand with the palm facing
up.
Perform measurements in a quiet and relaxed environment at
room temperature.
Do not move or shake the device during a measurement. Please
keep quiet and do not talk during measurements.
Proper cuff size is critical for accurate measurements. Follow the
instructions in this manual and printed on the cuff to ensure the
appropriate size of cuff is being used.
Keep in mind that blood pressure naturally varies from time to
time throughout the day and is affected by lots of different factors
such as stress, eating, smoking, alcohol consumption, medication,
and physical activity, etc.
Normally the blood pressure rises while at work and is at its lowest
during sleeping period.
Blood pressure measurements should be interpreted by a
physician or a trained health professional who is familiar with your
medical history. Using the unit and recording the results regularly
for your physician to interpret, you will keep your physician
informed of the continuing changes in your blood pressure.
If you have one of the circulatory problems as arteriosclerosis,
diabetes, liver disease, kidney disease, severe hypertension,
peripheral circulation….., please consult your healthcare
professional before using the device.
This product is not suitable for people with arrhythmias and
pregnant women.
Blood pressure measurements taken with this device are
equivalent to those obtained by a trained observer using the cuff /
stethoscope auscultation method and are within the accuracy
limits prescribed by the American National Standard for Manual,
electronic, or Automated Sphygmomanometers
Precautions
Precautions
*
Do not use this manual and product as a substitute for advice,
diagnosing or treating a health problem or prescribing any
medication by your doctor. If you have a medical problem,
promptly consult your healthcare provider.
*
Read the Instruction Manual thoroughly before measuring and
keep it at hand for your reference at any time.
*
This device uses the oscillometric method to measure systolic
and diastolic blood pressure as well as your heart rate. It’s
recommended for use by people over the age of 18 and not to be
used on infant or children.
*
The device is designed for home use and not suitable for clinical
use.
*Attention
!
1. Do not use the device on infants, children, or those who cannot express their
own intention.
2. The device is equipped with sensitive electronic components. While
measuring, avoid strong electrical or electromagnetic fields, e.g. mobile
phones, microwave ovens, etc; or it may lead to temporary reading error or
inaccuracy.
3. To avoid accidental strangulation, keep this product away from children and do
not drape tube around neck.
4. Consider the electromagnetic compatibility of the device (ex. power
disturbance, radio frequency interference etc.) Please use it indoor only.
5. Over high frequency measurements may result in blood flow interference,
which is likely to cause uncomfortable sensations, such as partial
subcutaneous hemorrhage, or temporary numbness to your arm. In general,
these symptoms should not last long. However, if you do not recover in time,
please seek your medical practitioners for help.
7 8
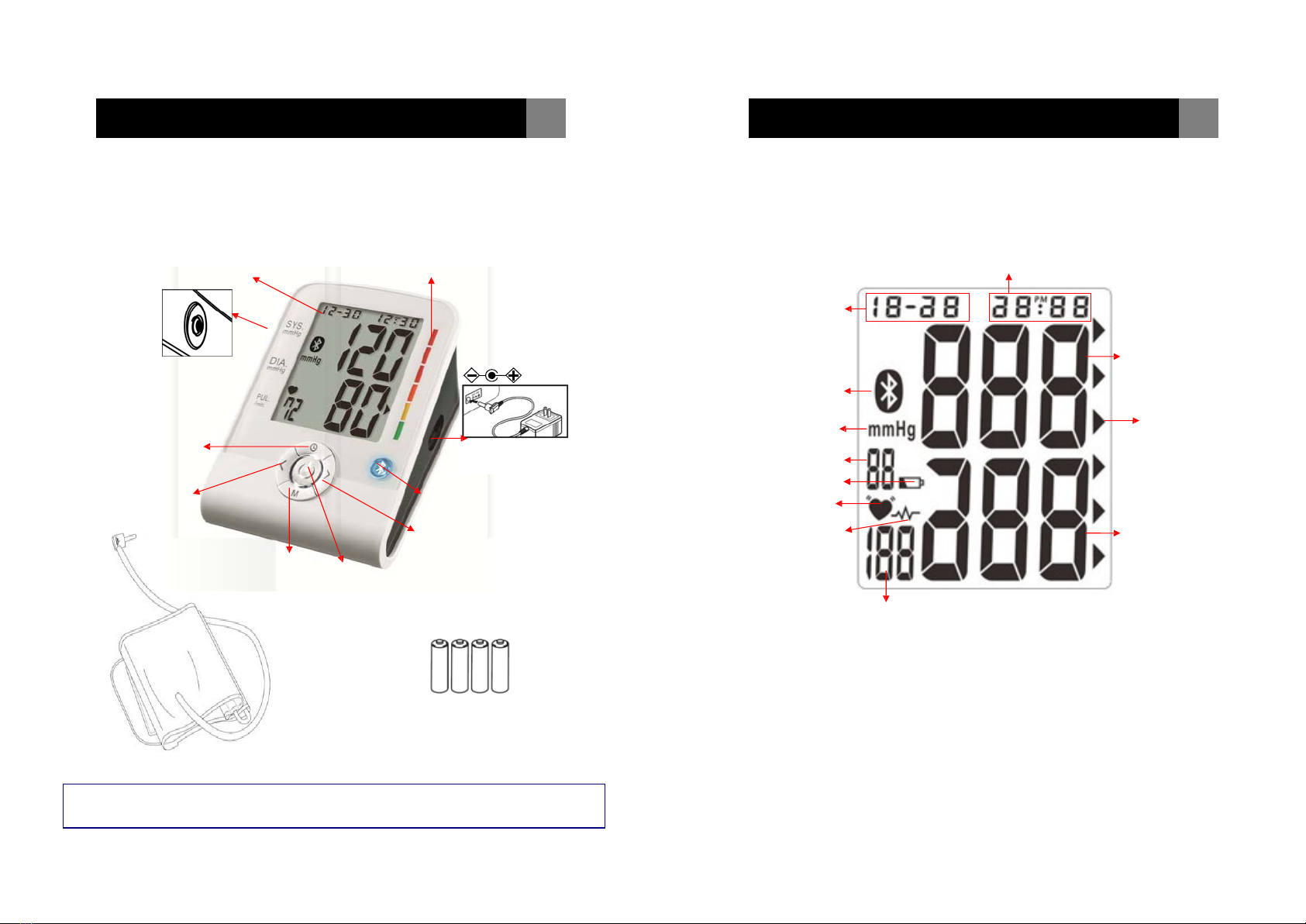
Part names and product components Unit display
LCD
DISPLAY
RISK CATEGORY INDICATOR Hour : Minute
(Also represents YEAR in Setting Mode)
Month / Date
Arm Cuff hole DC JACK Systolic Rate
Bluetooth Symbol
MODE
BUTTON
DOWN
BUTTON
MEMORY
BUTTON
6V 1A AC Adapter
(Excluded)
BLUETOOTHINDICATOR
UP
BUTTON
START / STOP
BUTTON
Blood Pressure
Unit
Memory Record Number
Low Battery Symbol
Pulse Symbol
Irregular Heartbeat
Detector
Heart Rate
Risk Category Indicator
Diastolic Rate
ARM CUFF WITH TUBE
AAA “LR03” (1.5V)
ALKALINE BATTERY X 4
*Caution
!
Substitution of a component different from that supplied might result in measurement error.
Device Overview
Device Overview
9 10

BP Category Indicator
This device is equipped with BP Category Indicator which classified
the blood pressure results with WHO (World Health Organization) BP
Classifications, which are Sever Hypertension, Moderate
Hypertension, Mild Hypertension, High Normal, Normal, and Optimal.
The Corresponding LCD segment will be turned on along with the
systolic, diastolic, and pulse rate information.
Each six segments of the bar indicator corresponds to the WHO blood
pressure classification described on the below:
WHO Blood Pressure Classification
* Reference Material: Jourmal of Hypertension 1999, Vol 17 No. 2.
*Note!
When a person’s systolic and diastolic pressures fall into different categories, the higher
category should apply. Examples listed as below:
Moderate Hypertension
High Normal
Normal
Optimal
Stages of Blood Pressure Levels
Systolic
(mmHg)
Diastolic
(mmHg)
Grade 3
Severe Hypertension
≧
180
≧
110
Grade 2
Moderate Hypertension
160 ~ 179
100 ~ 109
Grade 1
Mild Hypertension
140 ~ 159
90 ~ 99
High-Normal
130 ~ 139
85 ~ 89
Normal
120 ~ 129
80 ~ 84
Optimal
< 120
< 80
*Note!
Please note that other risk factors (e.g. diabetes, obesity, smoking, etc.) need to be
taken into consideration and may affect these figures. Consult with your physician for
an accurate assessment.
Features
Symbol Definitions
SYMBOLS Definitions
Low Battery Symbol
This symbol appears when the battery power is excessively
low or the polarity reverses.
We suggest you replace all batteries with new ones, and
make sure the +/- polarities are properly positioned.
Pulse Symbol
Once pulse is detected, the symbol flashes with each pulse
beat.
Our suggestion:
Please do not talk or move during measurements.
Irregular Heartbeat
Detector
This symbol appears for 1 minute when the user was talking,
moving, shaking, or an irregular heart beat was detected
during measurements.
Our suggestion:
Please do not talk or move during measurements.
Repeat the measurement after resting for at least 5 minutes,
and restart your measurement while sitting down comfortably
and quietly.
Bluetooth Symbol
LCD displays this symbol when Bluetooth Function turns ON.
Risk Category Indicator
Bar
The arrowhead points out the specific Risk Category that your
measurement reading fits in.
11 12

For adults 18 and older who are not on medicine for high blood
pressure, are not having a short-term serious illness, and do not
have other conditions, such as diabetes and kidney disease. To
determine category of risk when systolic and diastolic readings fall
into two areas, use the higher of the two numbers for
classification. There is an exception to the above definition of high
blood pressure for people with diabetes and chronic kidney
disease. A blood pressure of 130/80 mmHg or higher is considered
high blood pressure for those individuals.
Irregular Heartbeat Detector
The symbol will appear on screen indicating a certain
heartbeat irregularity was detected during measurement.
The heartbeat rhythm that is more than or less than 25% from the
average rhythm is usually defined as an irregular heartbeat rhythm.
Talking, moving, shaking or an irregular pulse during the
measurement can result in the appearance of this symbol.
Usually this is not a cause for concern, however if the symbol
appears often, we recommend you seek medical advice.
And please note that the device does not replace a cardiac
examination, but serves to detect pulse irregularities at an early
stage.
*Note
!
The pulse display is not suitable for checking the frequency of heart
pacemarkers. If a certain pulse irregularity is detected during
measurement often, we recommend you seek medical advice
As a safeguard, we recommend that if you have arrhythmias such as
atrial or ventricular premature beats and atrial fibrillation or any other
special conditions you should check with your physician before using
your device.
The IHB function is not designed for use by people with arrhythmias nor
for diagnosing or treating an arrhythmic problem. In order to filter the
unstable status of user and avoid affecting the detection of heart rate
from any movement, shaking or talking in the beginning of
measurement, the method of averaging heart beat intervals of subject
device is calculated with the three proper heart beat pulses detected in
the beginning of measurement and that is different from a strict
mathematical averaging of all recorded intervals.
At least 3 beats with at least 25% difference from the average heart
beat interval will generate the IHB icon on the screen.
Features
Features
*Note !
The above table is not exact for classification of blood pressure and it's
intended to be used as a guide in understanding non-invasive blood
pressure measurements.
Usually this is not a cause for concern; however we recommend you
consult with your physician for proper diagnosis or seek medical advice.
Please note that thedevicedoes not appropriate to diagnosehypertension,
and it is only for user reference on blood pressure monitoring.
13 14
Bluetooth Communication Function
HL858DC features a built-in Bluetooth Communication function,
which enables the device automatically transmit measuring results to
paired Bluetooth device. When connection established, BPM would
transmit memory data such as Measure Date, Systolic Pressure,
Diastolic Pressure and Pulse Rate to the Bluetooth device.
HL858DC also supports user proceed with Bluetooth device to
perform Date/Time Synchronization, Measurement Synchronization,
User Selection Synchronization, History Data Transmission, and
Memory Delete.
If paired Bluetooth device is not working or is not within RF range of
this device, the measuring results will be stored in the blood pressure
monitor's memory. For more details, please refer to “Bluetooth
Communication” page.
Bluetooth compatibility with blood pressure monitor
for
Bluetooth-enabled device is:
Bluetooth 4.0 for Android 4.3 or above,
Bluetooth 4.0 for iOS 7.0 or above
When LOW BATTERY SYMBOL appears on the display, or no
reaction toward operation, please change batteries.
Replace all worn-out batteries with new ones and do not mix new and
used batteries. Do not mix alkaline, standard (carbon-zinc) or
rechargeable (cadmium) batteries either. Such action may shorten
the battery life or cause the device to malfunction.
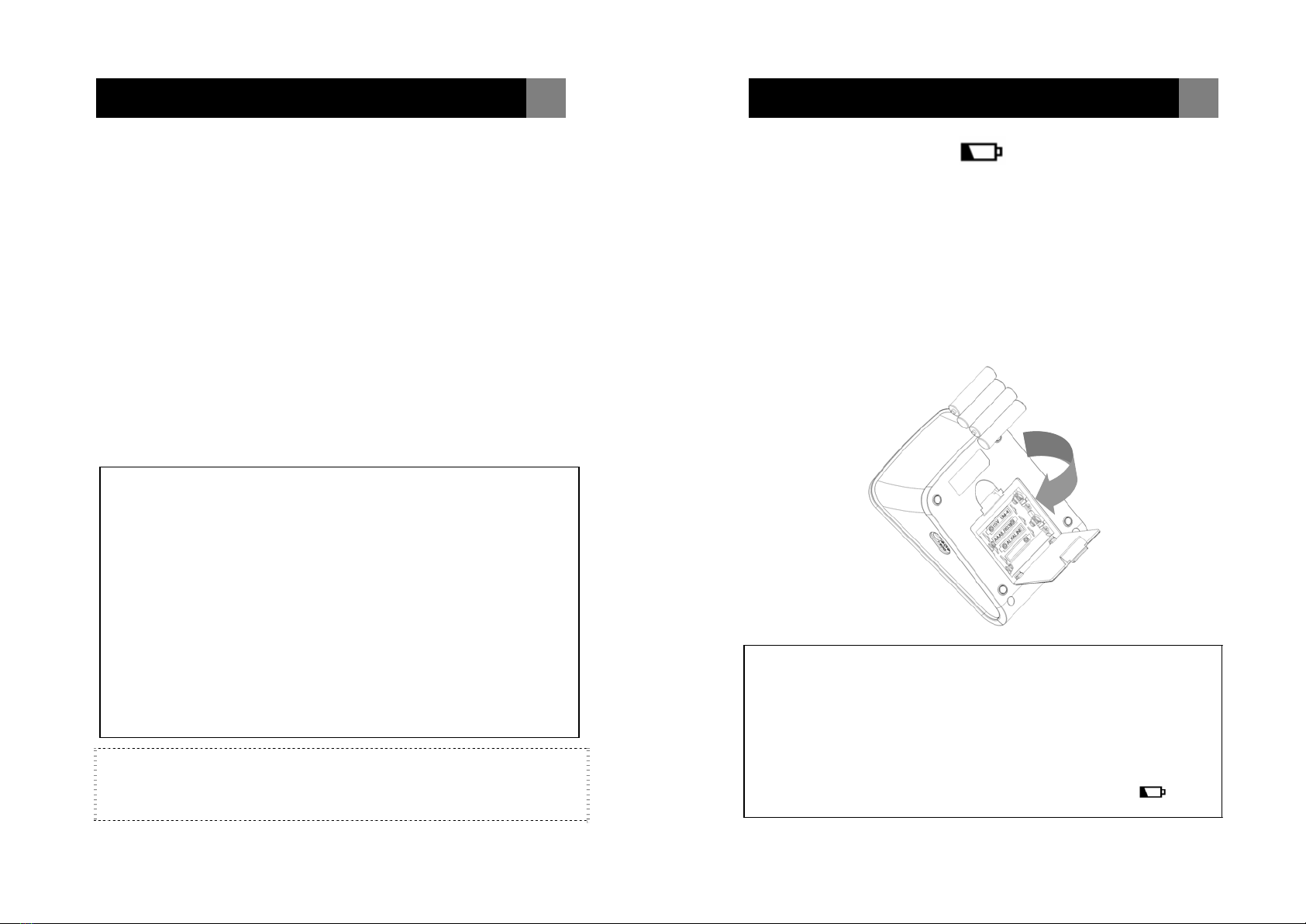
Slide the battery cover and insert 4 AAA (LR03) alkaline batteries into
the battery compartment as shown on the figure below. Make sure
the polarities “+” and “–” ends are properly positioned.
*Attention
!
Batteries are hazardous waste. Do not dispose of them together with the
household
garbage. Please discard worn-out batteries to the recycling site according to
local
regulations.
Keep the battery away from children in case they choke on it.
To prolong the battery life and prevent damage caused by leakage, remove the
batteries from the device if the device is not to be used for a long period.
The device will keep the last measuring results after changing batteries, please reset
date and time.
Please replace all worn-out batteries with new ones when you are operating the
Bluetooth communication function, and the LOW BATTERY SYMBOL
on the display.
appears
Installing Batteries
Features
AboutBluetoothCommunicationFunction
The Bluetooth communication function might not be workable to some
Bluetooth devices because of the compatibility of Android system.
Some issues that the Bluetooth implementations on these devices have unresolved
errors. It is not because of the Bluetooth module in monitor is not supported.
*Note
!
HL858DC is subject to and complies with electromagnetic compatibility
(EMC) standard of EN 60601-1-2, EN 301 489-1, EN 301 489-17, EN 300
328 and U.S. federal guidelines, Part 15 of the FCC (Federal
Communications Commission) rules for devices with RF capability. These
guidelines help ensure that your device will not affect the operation of
other nearby devices. Additionally, other devices should not affect the use
of your device.
Other wireless devices that are in use nearby, such as a cell or mobile
phone, or a wireless network, may prevent or delay the transmission of
data from your device to paired Bluetooth device. Moving away from the
source of the interference or turning off these devices to resolve the
problem.
Make sure HL858DC and paired Bluetooth device are within acceptable
distance (no more than 10 meters) with each other. If not, put them closer.
If you plan to transmit test results to paired Bluetooth device, be sure to
select User 1, 2, or 3 before measurements, in case other people’s results
may be transmitted to your paired Bluetooth device or included in your
past results.
15 16

This monitor is designed for operation with batteries or an AC adapter.
Please use only a compatible AC adapter with required voltage and
current as indicated in this manual.
Press your brachial artery approximately 1 inch (2 ~ 3 cm) above
the elbow on the inside of your left arm to determine where your
strongest pulse is.
Slide the end of arm cuff furthest from the tube through the metal
ring to a loop. The smooth cloth should be on the inside of the cuff.
If the cuff is located correctly, the velcro will be on the outside of
the cuff and metal ring will not touch your skin.
Put left arm through the cuff loop.
The bottom of the cuff should be approximately 1 inch (2 ~ 3 cm)
above the inner elbow. The tube should lie over the brachial artery
on the inner part of the arm.
Pull the cuff so that the top and bottom edges are tightened
around your arm.
When the cuff is positioned properly, press the velcro firmly
against the pile side of the cuff.
Sit on a chair and lay your forearm on the table so that the cuff is
at the same level as your heart.
Relax your arm and turn your arm
upward.
Make sure there are no kinks in the air
tube.
*Note
!
Fit the cuff snugly, leaving enough space for 1 inch (2 ~ 3 cm) between the inner
elbow and the lower edge of the cuff, or the measurement may not be accurate.
This monitor comes with one size of arm cuff: 9” ~ 13” (23 ~ 33 cm).
In case the cuff kept pumping up non-stop, open the cuff at once.
Do not wrap the cuff around any body part other than your arm.
The device is not supposed to be used when your arm is wounded or injured.
Applying the Cuff
Using the AC Adapter
*Note
!
•
No batteries are needed when operating with an AC adapter.
•
Please unload the batteries when operating with an AC adapter for
an extended period of time.
•
Leaving the batteries in the compartment for a long time may
cause leakage, which may lead to damage of the unit.
•
Recommend Adapter specification, do not use otherwise:
Input: 100 ~ 240V, AC, 50 ~ 60 Hz
Output: 6V, DC, 1A,
*Note
!
When you use the blood pressure monitor with AC adapter, do not
position the device to make it difficult to disconnect the adapter plug.
17 18

A.
Press
button ("YEAR" flashes). Press
or
button
to adjust YEAR value.
B.
Press
button ("MONTH" flashes). Use
or
button
to adjust MONTH (1, 2, 3,……, 12).
C. Adjust DATE (1, 2, 3,…, 31), HOUR (1, 2, 3,……..12PM,1 PM,…, 12)
and MINUTE (00,01,02,03,.....59) as described in Step A above.
D. When settings are done, press button to confirm
the settings. The device turns to standby mode.
Turning Bluetooth Feature ON/OFF
User can press and hold button 3 seconds to turn the
Bluetooth feature ON/OFF in Standby Mode.
B.
With the cuff wrapped around your upper arm,
press
button to start measurement.
All display units appear on the screen.
After all symbols disappear, the display will show
“00”. The monitor is “Ready to Measure” and will
automatically inflate to the level that is right for
you.
C.
After inflation of the cuff, the pressure will
slowly decrease. When pulse is detected,
PULSE SYMBOL flashes.
*Note
!
If the cuff does not stop inflating, remove the cuff at once.
To stop measurement, press button. The cuff will deflate immediately
after the button is pressed.
Bluetooth feature ON Bluetooth feature OFF
Taking a Measurement
A. Before measurement, press
or
button to select User 1,
2, or 3.
D.
LCD screen displays
your
systolic rate, diastolic rate,
pulse, Risk Category Indicator
Bar, and Irregular Heartbeat
Detector symbol (if any) with
date and time for 1 minute.
(Year and Date / Time display
alternate automatically)
E.
Without any operation for 1 minute, device automatically shuts
off.
*Note
!
The Bluetooth Feature Switch default setting is ON
Once Bluetooth Feature turns ON, the LCD appears Bluetooth symbol in any mode.
*Note
!
Do not inflate the cuff until it is wrapped around your upper arm.
Measurement Procedure
Measurement Procedure
Switch on the Monitor
A. Press
button to switch on the monitor.
B. All segments appear on the screen.
Setting Year, Date and Time
19 20

Storing data
After each measurement, the systolic and diastolic pressure, heart
rate, pulse, Risk Category Indicator Bar, and Irregular Heartbeat
Detector symbol (if any) with date and time will be automatically
stored.
The monitor can store up to 120 memories for 3 users, and
automatically replace the oldest data with new one.
Bluetooth Communication
To actually perform the Bluetooth Communication, please follow above
steps:
1. To activate Bluetooth function, please make
sure your Bluetooth device have downloaded
the software application (DailyChek®), and
follow pairing instruction.
2. Turn on Bluetooth function of your Bluetooth
device beforehand (For example: mobile
phone).
3. When connection established, HL858DC will
light Bluetooth indicator if it’s in a reachable
range (no more than 10 meters) with each other.
Bluetooth Indicator
lit constantly
Recalling data
A.
Press
or
button to select User 1, 2, or 3.
B.
Press M button to enter Memory Mode.
LCD displays average of last 3 measuring results
first.
C.
Press M button again, LCD displays the latest
measuring result. Use
or
button to
scroll through all stored measuring results.
(Year and Date / Time display alternate
automatically)
D.
To stop reading memories, press button,
and switch to Standby Mode.
Erasing data
A.
Press
or
button to select User 1, 2, or 3.
B.
Press M button to enter Memory Mode.
A. Date/Time, Measurement and User
Selection Synchronization
The BPM’s Date/Time Setting and User Selection can be synchronized by
Bluetooth device which have downloaded the software application.
To start measurement, please follow below steps:
1. The BPM press button to taking measurement.
2. The BPM and the Bluetooth device display the current cuff pressure
simultaneously.
3. When measurement completed, the BPM displays measurement
result, and the Bluetooth device display the same result as BPM.
B. Memory Delete
To delete User (1, 2, or 3) memory data or all memory
data in the BPM; you may use Bluetooth device which
have downloaded the software application to
complete the deletion.
C. History Data Transmission
Under Standby Mode, the BPM received the request
from Bluetooth device, and the BPM will transmit history data in memory
to Bluetooth device.
Please retry the above steps to transmit history data to other Bluetooth
device.
C.
Press and hold
and
buttons at the same
time, the data will be erased automatically.
D.
To confirm the data in the selected user has been erased, press
M button and no data should appear.
Note: Once deleted, your data can NOT be restored.
Memory Function
*Note
!
Without any operation in 1 minute, the device shuts off automatically
and Bluetooth Transmission OFF.
Standby Mode: Segmentsappeared, butnot under BPM measuring or
datatransmitting.
Sleeping Mode: Clear all LCD segments.
HL858DC can only pair up with one Bluetooth device at a time.
21 22

General Use
Do not in any way twist the cuff.
Do not press button if the cuff is not wrapped around
your upper arm.
Do not drop the product and avoid any strong impacts.
Maintenance
Use a piece of cloth with water or mild cleansing agent to wipe the
device and dry it immediately with a dry cloth.
Do not use detergent or any strong chemicals to clean the device.
Use only a dry cloth to wipe the cuff.
Do not attempt to disassemble or change any parts of the monitor,
including arm cuff, due to substitution of a component different
from that supplied might result in measurement error.
If any suggestion or service is requested, please consult your
service station.
Storage
If the device is not to be used for a long time, please remove the
batteries from the device (leaking of battery acid can cause the
device to malfunction).
Always store the unit in the storage case after use.
Do not place the device directly under sunlight, in high
temperature, or in humid or dusty places.
Do not store the device in extremely low (less than –13 ℉/–25 ℃)
and high (more than 158 ℉/70 ℃) temperature, nor in a place its
humidity exceeds 93% R.H.
Troubleshooting
Storage and Maintenance
SYMBOLS/SYMPTOMS
CONDITIONS/CAUSES
INDICATION/
CORRECTION
Unit does not turn on when
button is pushed.
Worn-out batteries.
Replace them with 4
new AAA (LR03)
alkaline batteries
Battery polarities have been
positioned incorrectly.
Re-insert the
batteries in the
correct positions.
Measuring Error Symbol
appears when blood
pressure value displayed is
excessively low or high.
Cuff has been placed incorrectly.
Wrap the cuff
properly so that it is
positioned correctly.
Did you talk or move during
measurement?
Measure again. Keep
arm steady during
measurement.
Shaking of the arm with the cuff
on.
Measuring Error Symbol
Air circuit abnormality. Cuff tube
may not be plugged into monitor
correctly.
Check cuff
connection. Measure
again.
Measuring Error Symbol Inflation pressure exceeding 300
mmHg.
Switch the unit off,
then measure
again.
Measuring Error Symbol
Error determining measurement
data.
Measure again.
BPM cannot communicate
with Bluetooth device
Paring has not been completed.
Please re-pairing the
BPM and Bluetooth
device with each other.
Bluetooth function is not turn on.
Please refer to
Page 17
“Measurement
Procedure” and Page
20 “Bluetooth
Communication” to
turn on the Bluetooth
function.
The distance between BPM and
Bluetooth device is out of
transmitting range.
Please make sure the
acceptable distance
(≦10 meters) with
each other.
Use an incompatible Bluetooth
device.
Please refer to
Page 13 “Bluetooth
compatibility” &
Page 26
"RF Specification”
Use non-Bluetooth device.
Unexpected loss of
electrical/mechanical integrity.
Re-insert the batteries
and try again.
Return the device to
your local distributor
or importer.
Note: If "EP" appears on the display, just return the device to your local distributor or
importer.
23 24

Warranty For One Year from Manufacturing Date
Please note that this warranty does not cover damage caused by
misuse or abuse; accident; the attachment of any unauthorized
accessory; alteration to the product; improper installation;
unauthorized repairs or modifications; improper use of
electrical/power supply; loss of power; dropped product; malfunction
or damage of an operating part from failure to provide
manufacturer’s recommended maintenance;transportation damage;
theft; neglect; vandalism; or environmental conditions; loss of use
during the period the product is at a repair facility or otherwise
awaiting parts or repair; or any other conditions whatsoever that are
beyond the control of importers or distributors.
Recalibration Notice
To ensure continued measurement precision, all digital blood
pressure monitors require recalibration regularly.
After 2 years from the manufacturing date, we recommend you have
your monitor recalibrate at the local distributor or importer. The
recalibration service plus the charge of shipping and handling fee
shall be charged accordingly.
*The contents of this manual and the specifications of the device
covered by this manual are subject to change for improvement
without notice.
Specifications
Warranty & Recalibration
Model Number HL858DC
Measurement
Method
Oscillometric
Rated Range of
Cuff Pressure
0 ~ 300 mmHg
Rated Range of
Determination
40 ~ 280 mmHg
Measurement
Range of Heart
Rate
40 ~ 199 Beats/minute
Accuracy Pressure: ± 3 mmHg
Pulse: ± 5 % Max.
Inflation
Automatic Inflation (Air Pump)
Deflation
Automatic Air Release Control Valve
Display
Liquid Crystal Display
Memory
120 Memory Total for 3 Users
Unit Dimensions
97.92 X 139.95 X 56.75 mm (L X W X H)
3.86 X 5.51 X 2.23 inch (L X W X H)
Unit Weight
291 g ± 10 g
(
10.26 oz ± 0.35 oz
)
(Cuff & Batteries Excluded)
Cuff Size
23 ~ 33 cm (approx. 9 ~ 13 inch)
Storage/
Transportation
Environment
Temperature: -25 °C ~ 70 °C (-13 °F ~ 158 °F)
Humidity: ≤93 % R.H.
Operation
Environment
Temperature: 5 °C ~ 40 °C (41 °F ~ 104 °F)
Humidity: 15 % ~ 93 % R.H.
Power Supply
1.
AAA “LR03” (1.5V) alkaline battery x 4
2.
6V 1A AC Adapter (Excluded)
Battery Life Approx. 200 Measurements (Bluetooth ON)
Sleeping Mode
Without any operation for 1 minute, device
automatically shuts off.
Accessories
4 AAA (LR03) Alkaline Batteries, Arm Cuff with
Tube, Instruction Manual, Storage Pouch
25 26

Follow instructions for use.
BF Classification:
-Internally powered equipment
-BF type applied part -IP22
-Not suitable for use in presence of flammable anesthetic mixture with air or with
Oxygen or nitrous oxide
-Continuous operation with short-time loading
To avoid inaccurate results caused by electromagnetic interference between
electrical and electronic equipment, do not use the device near a mobile phone or
microwave oven. At least keep a maximum output power of 2 W yields and distance
3.3m away from this equipment.
Discard the used product to the recycling collection point according
to local regulations.
Manufacture: HEALTH & LIFE Co., Ltd.
9F, No. 186, Jian Yi Road, Zhonghe District, New Taipei City, Taiwan
www.healthandlife.com.tw
Serial Number
Note
Specifications
RF Type
Bluetooth 4.0 BLE
RF Modulation
GFSK
Data Throughput
0.2Mbps
Expected Delay (Latency
Range) in Wireless (RF)
Communication
The latency time is less than 0.3ms second
from sender to receiver.
Integrity Channel Quality-Driven Data Rate (CQDDR)
technology increases the effective data rate
and integrity in noisy environments.
Security
AES-128 and application layer user defined
Wireless Operation
Distance
Class 2 (Maximum: 10 meter)
RF Frequency / Need for
Spectrum Management
2402 - 2480 MHz
(allowing for guard bands)
Maximum Limitation
Unlimited
Maximum Permitted
Power
2.5 mW
Proximity of Other
In-band Transmitters
Used in Vicinity
up to 40 bands (2 MHz spacing; centered
from 2402 to 2480 MHz)
Wireless Communication
Profile
GATT – Client and Server
Wireless Coexistence
Support for 802.11 Coexistence
System requirement of
the Bluetooth device
Android 4.3 or above, iPhone 4S or above
27 28

Appendix
Guidance and manufacturer’s declaration – electromagnetic emissions
The device is intended for use in the electromagnetic environments listed below, and should only be
used in such environments:
Emissions test Compliance
Electromagnetic environment –
guidance
RF emissions
CISPR 11
Group 1
RF energy is used only to maintain device’s
operation. Therefore, its RF emissions are so
low that it’s not likely to cause any
interference in nearby electronic equipment.
RF emissions
CISPR 11
Class B
The device is suitable for use in all
establishments, including domestic
establishments, and those directly connected
to the public low-voltage power supply
network
that supplies buildings used for domestic
purposes.
Harmonic emissions
IEC 61000-3-2
Class A
Voltage fluctuations/
flicker emissions IEC
61000-3-3
Complies
Guidance and manufacturer’s declaration – electromagnetic immunity
The device is intended for use in the electromagnetic environments listed below, and should only be
used in such environments:
HL858DC essential performance per IEC 80601-2-30 additional
essential performance requirements:
201.12.1.102 Limits of the error of the manometer from
environmental conditions
Over the temperature range of 5 °C to 40 °C (41 °F ~ 104 °F ) and
the relative humidity range of 15 % to 93 %(non-condensing), the
maximum error for the measurement of the CUFF pressure at any
point of the NOMINAL measurement range shall be less than or
equal to ± 3 mmHg (± 0.4 kPa) or 2 % of the reading, whichever is
greater.
201.12.1.107 Limits of the change in error of the blood
pressure determination
The laboratory limits of the change in error of the BLOOD PRESSURE
DETERMINATION of the AUTOMATED SPHYGMOMANOMETER shall be
less than 3 mmHg (0.4 kPa).
Note
*Note
!
This equipment has been tested and found to comply with the limits for a Class B digital device, pursuant to Part
15 of the FCC Rules. These limits are designed to provide reasonable protection against harmful interference in
a residential installation.
This equipment generates uses and can radiate radio frequency energy and, if not installed and used in
accordance with the instructions, may cause harmful interference to radio communications. However, there is no
guarantee that interference will not occur in a particular installation. If this equipment does cause harmful
interference to radio or television reception, which can be determined by turning the equipment off and on, the
user is encouraged to try to correct the interference by one or more of the following measures:
The user is encouraged to try to correct the interference by one or more of the following measures:
Reorient or relocate the receiving antenna.
Increase the separation between the equipment and the receiver.
Connect the equipment into an outlet on a circuit different from that to which the receiver is connected.
Consult the dealer or an experienced radio/TV technician for help.
CAUTION:
To assure continued FCC compliance:
1. Any changes or modifications not expressly approved by the grantee of this device could void the user's
authority to operate the equipment.
2. This equipment complies with FCC radiation exposure limits set forth for an uncontrolled environment.
FCC Label Compliance Statement:
This device complies with Part 15 of the FCC Rules. Operation is subject to the following two conditions:
(1) this device may not cause harmful interference, and
(2) this device must accept any interference received, including interference that may cause undesired
operation.
*Note
!
“Changes or modifications not expressly approved by the manufacturer could void the user’s authority to operate
the equipment”.
Immunity test
IEC 60601
test level
Compliance level
Electromagnetic environment –
guidance
Electrostatic
discharge (ESD)
IEC 61000-4-2
± 6 kV contact
± 8 kV air
± 6 kV contact
± 15 kV air
The relative humidity should be at least 5
%.
Power frequency
(50/60 Hz)
magnetic field
IEC 61000-4-8
3 A/m
30 A/m Power frequency magnetic fields should
be at levels characteristic of a typical
location in a typical commercial or
hospital environment.
Recommended separation distance
I
r =
────
(m)
188
where I is the current in amperes in a
power bus or an appliance wire and r is
the recommended separation distance
between your device and the power bus or
appliance wire, in meters (m).
Electrical fast
transient/burst
IEC 61000-4-4
±
2 kV for power supply
lines
± 1 kV for input/output
lines
±
2 kV for power supply
lines
± 1 kV for input/output
lines
Mains power quality should be that of a
typical commercial or hospital
environment.
Surge
IEC 61000-4-5
±
1 kV line(s) to line(s)
± 2 kV line(s) to earth
±
1 kV line(s) to line(s)
± 2 kV line(s) to earth
Mains power quality should be that of a
typical commercial or hospital
environment.
interruptions and
voltage variations
on power supply
input lines
IEC 61000-4-11
<5 % UT
(>95 % dip in UT)
for 0,5 cycle
40 % UT
(60 % dip in UT)
for 5 cycles
70 % UT
(30 % dip in UT)
for 25 cycles
<5 % UT
(>95 % dip in UT)
for 5 sec
<5 % UT
(>95 % dip in UT)
for 0,5 cycle
40 % UT
(60 % dip in UT)
for 5 cycles
70 % UT
(30 % dip in UT)
for 25 cycles
<5 % UT
(>95 % dip in UT)
for 5 sec
Mains power quality should be that of a
typical commercial or hospital
environment. If the user of the device
requires continued operation during
power mains interruptions, it is
recommended that the device be powered
from an uninterruptible power supply or a
battery.
29 30
Appendix
Recommended separation distances between portable and mobile RF communication equipment
and the device.
The device is intended for use in an electromagnetic environment where radiated RF disturbances are under control.
User can help prevent electromagnetic interference by keeping the device at a minimum distance from portable and
mobile RF communications equipment (transmitters). Below table details the maximum output power of
transmitter:
Rated maximum output power
of transmitter
W
Separation distance according to frequency of transmitter
m
150 kHz to 80 MHz
d = 1.2 80 MHz to 800 MHz
d = 1.2 800 MHz to 2.5 GHz
d = 2.3
0.01
0.12
0.12
0.23
0.1
0.38
0.38
0.73
1
1.2
1.2
2.3
10
3.8
3.8
7.3
100
12
12
23
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in metres (m) can be estimated using
the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts (W) according
to the transmitter manufacturer.
NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures,
objects and people.
Guidance and manufacturer’s declaration – electromagnetic immunity
The device is intended for use in the electromagnetic environments listed below, and should only be used in such
environments:
P/N:XXXXXXXXX VER:A001
YYYYMMDD
Blood Pressure Diary
□Before
Date:
Time:
Meal
□
After
Systolic / Diastolic
:
Pulse
:
□Before
Date:
Time:
Meal
□
After
Systolic / Diastolic
:
Pulse
:
□Before
Date:
Time:
Meal
□After
Systolic / Diastolic
:
Pulse
:
□Before
Date:
Time:
Meal
□After
Systolic / Diastolic:
Pulse:
□Before
Date:
Time:
Meal
□After
Systolic / Diastolic
:
Pulse
:
□Before
Date:
Time:
Meal
□After
Systolic / Diastolic
:
Pulse
:
□Before
Date:
Time:
Meal
□
After
Systolic / Diastolic
:
Pulse
:
□Before
Date:
Time:
Meal
□After
Systolic / Diastolic
:
Pulse
:
□Before
Date:
Time:
Meal
□After
Systolic / Diastolic:
Pulse:
□Before
Date:
Time:
Meal
□After
Systolic / Diastolic
:
Pulse
:
Immunity test IEC 60601 test
level
Compliance
level
Electromagnetic environment – guidance
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
3 Vrms
150 kHz to 80 MHz
3 V/m
80 MHz to 2.5 GHz
6 Vrms
3 V/m
Portable and mobile RF communications equipment should be used
no closer to any part of the device, including cables, than the
recommended separation distance calculated from the equation
applicable to the frequency of the transmitter.
Recommended separation distance
d = 1.2
d = 1.2 80 MHz to 800 MHz
d = 2.3 800 MHz to 2.5 GHz
where P is the maximum output power rating of the transmitter in
watts (W) according to the transmitter manufacturer and d is the
recommended separation distance in metres (m).
Field strengths from fixed RF transmitters, as determined by an
electromagnetic site survey,a should be less than the compliance
level in each frequency range.b
Interference may occur in the vicinity of equipment marked with
the following symbol:
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption
and reflection from structures, objects and people.
a Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM
and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed
RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the device is used
exceeds the applicable RF compliance level above, the device should be observed to verify normal operation. If abnormal performance is observed,
additional measures may be necessary, such as reorienting or relocating the device.
b Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
31 32
Table of contents
Other Health & Life Blood Pressure Monitor manuals

Health & Life
Health & Life HL858CC User manual

Health & Life
Health & Life HL858CC User manual

Health & Life
Health & Life HL858CB User manual

Health & Life
Health & Life HL158LD User manual

Health & Life
Health & Life HL158AU-BD User manual

Health & Life
Health & Life BPM280XL User manual

Health & Life
Health & Life HL858CG User manual

Health & Life
Health & Life HL858CB User manual