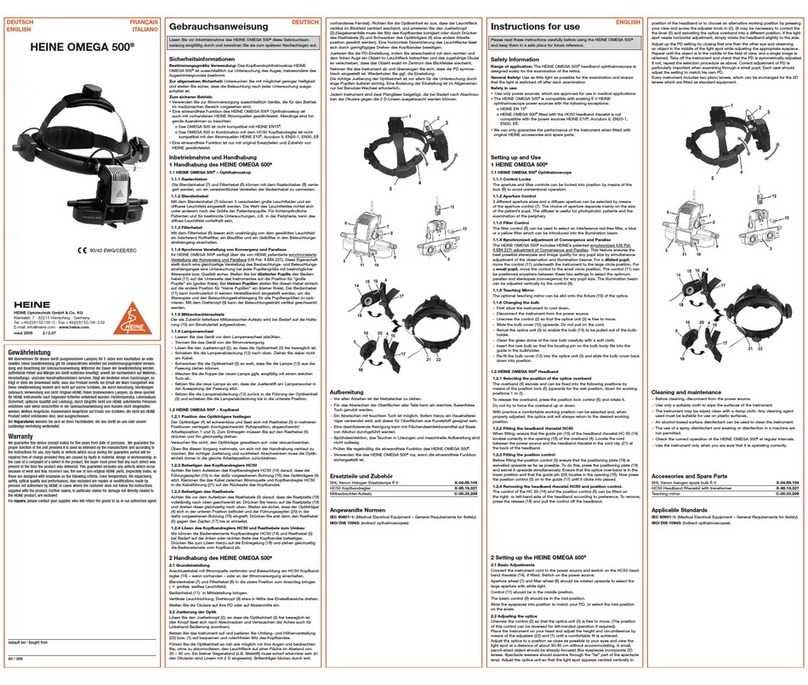
HEINE mini 3000 User manual

9/56
med 1212 2013- 09-10 med 1212 2013- 09-10
HEINE mini 3000®LED Dermatoscope
Please read and follow these instructions for use carefully and keep them for future reference.
General Conditions of Warranty
Instead of the statutory warranty time period of 2 years, HEINE will grant a guarantee of 5 years from the date
of the consignment of the goods ex works, concerning its equipment (excluding disposables, e.g. bulbs, single-
use articles, and rechargeable batteries). Date of consignment means that HEINE hands the goods over to the
transport carrier, freight forwarder or any other person designated by the Customer for the transport of the goods
without loading the collecting vehicle.
The guarantee covers irreproachable workmanship, on condition of the proper use of the equipment and the
observation of the operating instructions. During the warranty- and guarantee time period, errors and deficiencies
arising on the equipment will be rectified free of charge, in so far as such are evidenced by defective materials,
processing and/or constructional errors. Should buyer complain of a material deficiency during the warranty time
period, then the onus of proof is always to be on the orderer, that the product was defective already upon receipt
of the goods. The statutory warranty and the guarantee do not apply to loss or damage caused by wear and
tear, negligent use, the non-employment of original HEINE components and/or spares (in particular bulbs, as
these have been especially developed for HEINE instruments in accordance with the following criterions: colour
temperature, useful service life, safety, optical quality and performance. The statutory warranty and the guarantee
do not apply to interventions by persons not authorised by HEINE or when the operating instructions are not
observed by the customer.Any modification of a HEINE product with parts or additional parts which do not
conform to the original HEINE specification will invalidate the warranty for the correct function of the product
and further invalidate any warranty claims which result from such a change or modification. Further claims, in
particular claims for replacement of loss or damage, which are experienced otherwise than directly on the
HEINE product itself, are hereby excluded.
For U.S. only:
Caution: Federal law restricts this device to sale by or on the order of a Physician or Practitioner!
Intended Use
The HEINE mini 3000®LED Dermatoscope is a epiluminescence microscope (dermatoscope) for non-invasive
visual examination of intact skin (dermatoscopy) by medical professionals.
Warnings and Safety Information
CAUTION! Indicates potentially hazardous situations. Ignoring the corresponding instructions may lead
to dangerous situations of mild to moderate extent. (Background: yellow; Foreground: black)
NOTE! Indicates valuable advice in terms of installation, operation, maintenance or repair. Notes are
important, but not related to hazardous situations.
Product overview
1a Contact Plate
1b Small contact plate
2 Ocular
3 Focusing ring
4 Light port
5 Handle head
6 Slide switch 1/0
7 End cap
8 LED light source integrated
in instrument thread
ENGLISH
6
7
5
4
1b
1a
2
3
8

10/56 med 1212 2013 -09 -10
Setting up the HEINE mini 3000®LED Dermatoscope
To setup the HEINE mini 3000®LED Dermatoskope, please screw the instrument thread (8) onto the handle head
(5) (exclusively of the mini 3000 product line).To insert fresh batteries, unscrew the end cap (7) of the handle and
insert 2 new batteries (alkaline cells, size LR6/AA) with the plus contact facing the handle head (see picture).
To switch on the light source please slide the switch (6) down into position “1”. To switch off the light source slide
the switch up to position “0” or clip it firmly over your coat pocket.The handle is then automatically switched off.
Performance Indicator: When turning on the device the brightness will increase softly to 100% and will then
adjust to the brightness corresponding to the actual charge condition of the battery.
If you realize a significant drop of brightness or a flashing of the lightning immediately after switching on the
device, please change the batteries.
Operation of the HEINE mini 3000®LED Dermatoscope
Moisten the affected skin with HEINE dermatoscopy oil or comparable, e.g. with a cotton wool swab. Switch on the
device and place it gently over the lesion, so that it is in the center of the contact plate (1a).
The examiner’s eyes should be as close as possible to the ocular (2). With the free hand adjust the focusing ring
(3) until a clearly-focused image is obtained. Using the scale on top of the dermatoscope you can control the
adjustment of the focusing ring. In most cases it is only necessary to set up the focus once.
Removing the contact plate:
The contact plate (1a) is attached by a bayonet fitting.To remove, simply rotate the knurled ring counter-clockwise
and detach from the dermatoscope.The small contact plate (1b) can be used instead of the contact plate (1a) for
the examination of inaccessible lesions. It is a simple push-fit in the head. To remove it, simply hold the knurled
housing and pull off without twisting. When replacing, make sure that the light-port (4) faces the bulb/LED.
The HEINE mini 3000®LED Dermatoscope is not allowed to be used for eye examinations!
Cleaning and disinfection
The contact plate (1a) can be removed and sterilized or disinfected by any routine method e.g. in disinfectant
solution, by washing, boiling or autoclaving (up to 134 degree Celsius for 5min). Sterilization should be used only
after the examination of risk patients, because the durability of the contact plate will be reduced by sterilization.
The small contact plate (1b) and the dermatoscope can be cleaned by wiping with a damp cloth soaked in
alkaline or pH-neutral cleaning agents.
For disinfection of the outer surface of the instrument we recommend wipe disinfection using detergents, which
are released for medical devices.
Battery handle:
The handle can be cleaned with a damp cloth (e.g. alkaline or pH- neutral detergent). For disinfection of the outer
surface we recommend wipe disinfection by using detergents, which are released for medical devices made out
of plastic.
Disinfection by spraying or immersion as well as sterilization is not allowed and will damage the instrument!
Maintenance
The device does not require any regular maintenance.
Changing the light source:
With the HEINE mini 3000®LED Dermatoscope the LED cannot be changed.
Service
The device does not require regular service.
Do not open or modify the device. Repairs are only to be performed by qualified personnel.
Disposal
The product must be recycled as separated electrical and electronic devices. Please observe the relevant
state-specific disposal regulations.
11/56
med 1212 2013- 09-10 med 1212 2013- 09-10
Technical Specifications
Nominal voltage 3V DC
Nominal current typ. 220mA
Protection class Internal power supply
Application part Type BF
Device classification according to
IEC 62471:2006
Exempt
Battery Typ 2 Alkaline (1.5 V), size AA/LR6 or 1 HEINE mini 2Z
(rechargeable with HEINE®mini NT)
Magnification approx. 10x (depending on distance)
Focal Range (correction) approx. +/- 4dpt
Contact plates Silica glass, multi-coated
Environmental conditions
for operation
10°C to 35°C
30% to 90% rel. humidity
800hPa to 1060hPa
Environmental conditions
for storage and transport
-20°C to 50°C
10% to 95% rel. humidity
500hPa to 1060hPa
Dimensions (L x B x H)
for head and handle
165 x 52 x 35 mm³
Weight ca. 195g (incl. 2 batteries)
General Notes and Warnings
Check the correct operation of the device before use! Do not use the device if there are visible signs
of damage.
Do not use the device in hyperbaric chambers, in explosive or oxygen loaded environments!
Do not use the device near strong magnetic fields like MRI scanners!
Do not look into bright light sources by means of loupes. Hazard of glare!
The performance and safety of the device can only be guaranteed when fitted with original HEINE
accessories and HEINE spare parts. Otherwise the warranty is terminated.
Don’t modify the device! If the device is modified despite that, take care that use of the device is safe by
performing investigations and tests.
Your HEINE mini 3000®LED F.O. Dermatoscope is a precise optical instrument. Please handle the device
with care and clean it at regular intervals.
Store and use the device in dry and dust-free environments only! If you don’t use the device for a longer
period of time, please remove the batteries in advance.
Electromagnetic Compatibility
Medical electric devices are subject to special precautionary measures with regard to electromagnetic
compatibility (EMC). Portable and mobile high frequency communication equipment can affect medical
electric devices.
This device is intended for use by medical professionals in the electromagnetic environment specified
below. The user of this device should assure that it is used in such an environment.
The use of accessories, converters or cables other than the ones specified by HEINE might lead to increased
emission reduced electrical immunity of the medical equipment.
The device may not be stacked directly near or used directly beside other devices. If the device is to be
operated in a stack or with other devices, the device should be watched to ensure it operates properly in
this location.
12/56 med 1212 2013 -09 -10
Guidance and manufacturer’s declaration – electromagnetic emissions
The device is intended for use in the electromagnetic environment specified below.
The customer or the user of the device should assure that it is used in such environment.
Emission test Compliance Electromagnetic environment – Guidelines
RF emissions
CISPR11 Group 1 The device uses RF energy only for its internal function. Therefore, RF-emission
is very low and it is unlikely that any interference in nearby electronic equipment.
RF emissions
CISPR 11 Class B The device is suitable for use in all establishments, including domestic establish-
ments and those directly connected to the public low-voltage power supply
network that supplies buildings used for domestic purposes.
Warning: This device is intended only for use by medical professionals. This is a
device of class A CISPR 11 in the domestic environment, this device may cause
radio interference, so that it may be necessary in this case, to take appropriate
remedial measures, as e.g. orientation, new arrangement or shielding of the
device or restrict the connection to the site.
Harmonic Emissions
IEC 61000-3-2 Not
applicable
Voltage Fluctuations/
Flicker Emissions IEC
61000-3-3
Passed
Guidance and manufacturer declaration - Electromagnetic immunity
The device is intended for use in the electromagnetic environment specified below.
The customer or the user of the device should assure that it is used in such environment.
Immunity test IEC 60601 test level Compliance level Electromagnetic environment –
Guideline
Electrostatic discharge
(ESD) acc. to
IEC 61000-4-2
± 6 kV contact discharge
± 8 kV air discharge ± 6 kV contact discharge
± 8 kV air discharge Floors should be wood, concrete or
covered with ceramic tiles. If floors
are covered with synthetic material,
the relative humidity should be at
least 30 %.
Electrical fast transient/
burst
IEC 61000-4-4
± 2 kV for mains cables
± 1 kV for input and
output lines
± 2 kV for mains cables
± 1 kV for input and
output lines
The supply voltage quality should be
that of a typical commercial or hospital
environment.
Surge IEC 61000-4-5 ± 1 kV voltage
phase – phase,
± 2 kV voltage
phase – earth
± 1 kV voltage
phase – phase
± 2 kV voltage
phase – earth
Mains power quality should be that
of a typical commercial or hospital
environment.
Voltage dips, short
interruptions and voltage
variations on power
supply input lines
IEC 61000-4-11
< 5% UT, (>95% dip in UT)
for 1/2 period
40% UT, (60% dip in UT)
for 5 periods
70% UT, (30% dip in UT)
for 25 periods
<5% UT, (>95% dip in UT)
for 5 seconds
< 5% UT, (>95% dip in UT)
for 1/2 period
40% UT, (60% dip in UT)
for 5 periods
70% UT, (30% dip in UT)
for 25 periods
<5% UT, (>95% dip in UT)
for 5 seconds
Mains power quality should be that
of a typical commercial or hospital
environment. If the user of the device
device requires continued operation
during power mains interruptions, it
is recommended that the device be
powered by a UPS (uninterruptible
power supply) or a battery.
Power frequency
(50/60 Hz)
magnetic field
IEC 61000-4-8
3 A/m 3 A/m Power frequency magnetic fields
should be at levels characteristic of
a typical location in a typical
commercial or hospital environment.
Comment: UTis the a.c. supply voltage prior to application of the test level.

13/56
med 1212 2013- 09-10 med 1212 2013- 09-10
Guidance and manufacturer’s declaration – electromagnetic immunity
The device is intended for use in the electromagnetic environment specified below. The customer or the user of the device
should assure that it is used in such environment.
Immunity test IEC 60601 test level Compliance level Electromagnetic environment Guidelines
Conducted RF
IEC 61000-4-6 3 Veff
150 kHz to 80 MHz 3 V eff Portable and mobile RF communication equipment
should be used no closer to any part of the device,
including cables, than the recommended separation
distance calculated from the equation applicable to
the frequency of the transmitter.
Radiated HF
IEC 61000-4-3 3 V/m
80MHz to 2,5GHz 3 V/m Recommended separation distance:
d = 3,5/3 * SQRT (P/W)
d = 3,5/3 * SQRT (P/W) 80 MHz to 800 MHz
d = 7/3 * SQRT (P/W) 800 MHz to 2,5 GHz
where P is the maximum output power rating of the
transmitter in watts (W) according to the transmitter
manufacturer and d is the recommended separation
distance in metres (m).
Field strengths from fixed RF transmitters, as deter-
mined by an electromagnetic site surveyaa, should
be less than the compliance level in each frequency
range.b
Interference may occur in the vicinity of equipment
marked with the following symbol:
Note 1: At 80Hz and 800MHz, the higher frequency range applies.
Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and
reflection from structures, objects and people.
a
Field strengths from fixed transmitters, such as base stations for radio (cellular / cordless) telephones and land mobile
radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy.
To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be con-
sidered. If the measured field strength in the location in which the device is used exceeds the applicable RF compliance
level above, the device should be observed to verify normal operation. If abnormal performance is observed, additional
measures may be necessary, such as reorienting or relocating the device.
b
Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V / m.
Recommended separation distances for portable and mobile RF communication equipment and the device
The device is intended for use in the electromagnetic environment in which radiated RF disturbances are controlled.
The customer or user of the device can help prevent electromagnetic interference by maintaining a minimum distance
between portable and mobile RF communication equipment (transmitters) and the device as recommended below,
according to the maximum output power of the communications equipment.
Rated maximum output
power of transmitter (W) Separation distance according to frequency of transmitter (m)
150 kHz to 80 MHz
d = 3,5/3 * SQRT (P) 80 MHz to 800 MHz
d = 3,5/3 * SQRT (P) 800 MHz to 2,5 GHz
d = 7/3 * SQRT (P)
0.01 0.1 0.1 0.2
0.1 0.4 0.4 0.7
11. 2 1. 2 2.3
10 3.7 3.7 7.4
100 11. 7 11. 7 23.3
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in metres (m)
can be determined using the equation applicable to the frequency of the transmitter, where P is the maximum output power
rating of the transmitter in watts (W) according to the transmitter manufacturer.
Note 1: At 80 MHz and 800 MHz, the higher frequency range applies.
Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and
reflection from structures, objects and people.

14/56 med 1212 2013-0 9-10
The CE marking indicates that the product conforms to the provisions
of European Medical Device Directive (93/42/EEC).
Catalogue number
Manufacturer
Date of manufacture
Product bearing this symbol may not be disposed of together with
general household waste, but instead requires separate disposal.
Temperature limits in °C for storage and transport
Temperature limits in °F for storage and transport
Humidity limitation for storage and transport
Pressure limitation for storage and transport
Interference may occur in the vicinity of equipment marked with
the following symbol.
Fragile, handle with care!
Keep dry!
”Grüner Punkt“ (country-specific)
Please read and follow the instructions for use and keep them for
future reference (Background: blue; Foreground: white)
Type BF applied part
Explanation of utilized symbols
The following symbols are used on the device or on the packaging:
REF
Other manuals for mini 3000
4
Table of contents
Other HEINE Medical Equipment manuals

HEINE
HEINE HR User manual

HEINE
HEINE EN50 User manual
HEINE
HEINE GAMMA 2.1 User manual

HEINE
HEINE mini 3000 User manual

HEINE
HEINE BETA 200 LED User manual

HEINE
HEINE BETA 200 LED User manual

HEINE
HEINE XP User manual

HEINE
HEINE GAMMA 3.1 User manual

HEINE
HEINE mini 3000 User manual

HEINE
HEINE FlexTip + Laryngoscope User manual

HEINE
HEINE 3S LED HeadLight User manual

HEINE
HEINE S-Frame User manual

HEINE
HEINE i-View User manual

HEINE
HEINE GAMMA 2.2 User manual

HEINE
HEINE GAMMA Series User manual

HEINE
HEINE DELTA 20 User manual

HEINE
HEINE NC1 User manual

HEINE
HEINE visionPRO User manual

HEINE
HEINE iC1 User manual

HEINE
HEINE DELTA 30 User manual