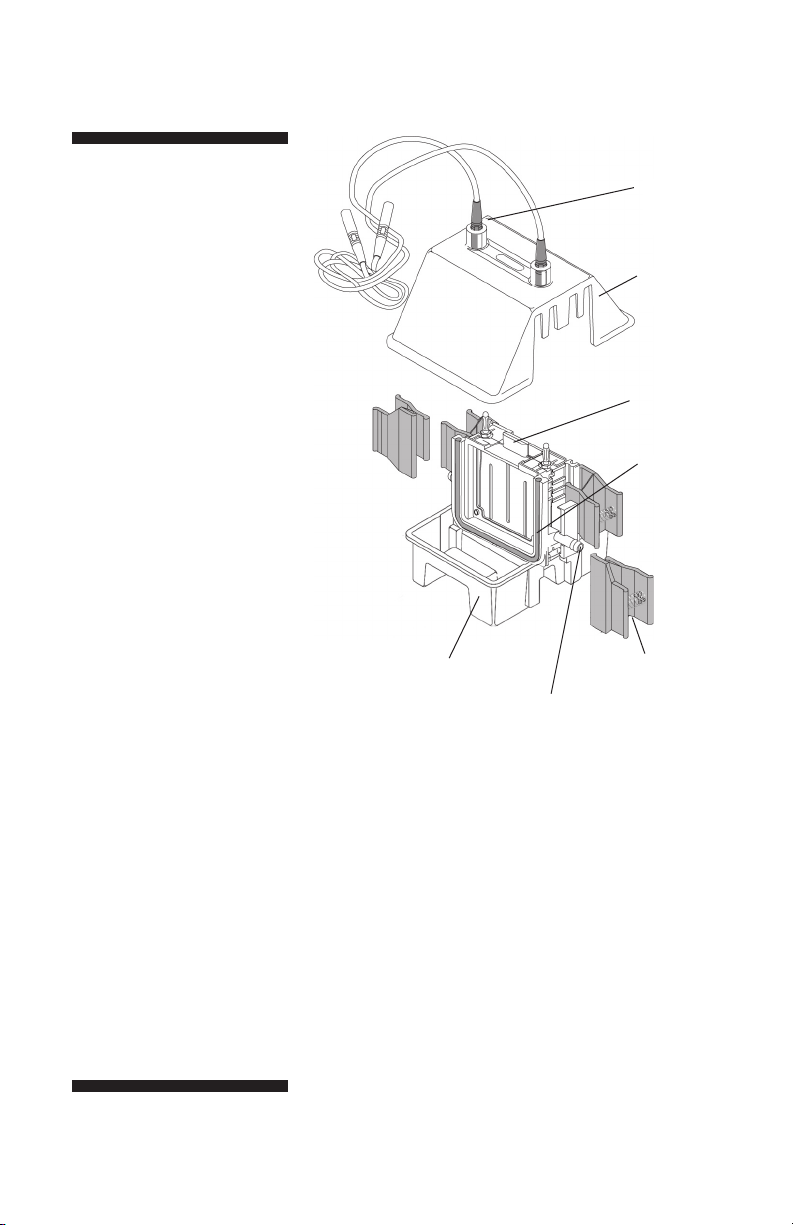
Hoefer SE 260 User manual
Other Hoefer Laboratory Equipment manuals

Hoefer
Hoefer HB1000 User manual

Hoefer
Hoefer UVC5000 User manual

Hoefer
Hoefer TE 70 User manual

Hoefer
Hoefer MacroVue UV-25 User manual

Hoefer
Hoefer TE 22 User manual

Hoefer
Hoefer SG500 User manual

Hoefer
Hoefer SE 400 User manual

Hoefer
Hoefer HE99X User manual

Hoefer
Hoefer TE70X User manual

Hoefer
Hoefer SG15 User manual
Popular Laboratory Equipment manuals by other brands

Belden
Belden HIRSCHMANN RPI-P1-4PoE installation manual

Koehler
Koehler K1223 Series Operation and instruction manual

Globe Scientific
Globe Scientific GCM-12 quick start guide

Getinge
Getinge 86 SERIES Technical manual

CORNING
CORNING Everon 6000 user manual

Biocomp
Biocomp GRADIENT MASTER 108 operating manual