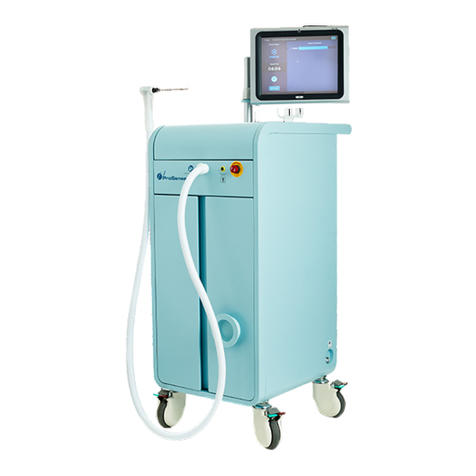
IceCure™Medical Ltd. DSR3200000 rev. G ProSense™
Confidential User Manual
3
Return to first page U.S User Manual
3.3.3 Flexible hose..................................................................................................43
3.3.4 Touch screen and User Manual activation.....................................................43
3.3.5 Cryohandle and cryoprobe.............................................................................45
3.3.6 Holder –Optional (Not available in some regions, e.g. China).....................47
3.3.7 Foot pedal (Not available in some regions, e.g. China).................................48
3.3.8 Temperature sensor (TS) ...............................................................................48
3.3.9 Dewar storage cases.......................................................................................50
3.4 Operational details ................................................................................................50
3.4.1 Starting the system.........................................................................................51
3.4.2 System pre-tests.............................................................................................52
3.4.3 Cryoablation procedure..................................................................................52
3.4.4 EXTRACTION step ......................................................................................52
4INSTALLATION AND SETUP .................................................................................53
4.1 Space and positioning requirements......................................................................53
4.2 Setup warnings and cautions.................................................................................53
4.3 Electrical requirements..........................................................................................54
4.3.1 For countries in which local line voltage is 100-127 VAC ...........................54
4.3.2 For countries in which local line voltage is 220-240 VAC ...........................54
4.4 Shipment components...........................................................................................54
4.5 Installation.............................................................................................................55
5OPERATING THE SYSTEM .....................................................................................56
5.1 Procedure Overview..............................................................................................56
5.2 Pre-operational stages ...........................................................................................56
5.2.1 Preparing the system for procedure ...............................................................56
5.2.2 Preparing the patient for procedure ...............................................................57
5.2.3 Switching on the ProSense™ cryoablation system .......................................58
5.2.4 Cryohandle.....................................................................................................59
5.2.5 General settings..............................................................................................59
5.2.6 Preparing the system for treatment................................................................63
5.2.7 Treatment Selection.......................................................................................81
5.3 Operational stages.................................................................................................87
5.3.1 Safe Operation in Percutaneous Procedures..................................................87
5.3.2 Preliminaries..................................................................................................87
5.3.3 Freeze Cycle ..................................................................................................89
5.3.4 Pause Option:.................................................................................................90
5.3.5 THAW ...........................................................................................................95
5.3.6 EXTRACTION process.................................................................................95
5.3.7 Show Last Treatment...................................................................................101
5.3.8 Replace (refill) the Dewar during a cryoprocedure .....................................102
5.4 Post-operational stages........................................................................................107
5.4.1 Removing the temperature sensor from tissue.............................................107
5.4.2 Removing the cryoprobe from the cryohandle ............................................107
5.4.3 Disassembling the support device - Hose-Holder........................................108
5.4.4 Disassembling the temperature sensor.........................................................108
5.4.5 Exiting the ProSense™ cryoablation system treatment mode.....................108
5.5 Instruct patients:..................................................................................................109
5.6 System failures....................................................................................................110
5.6.1 ProSense™ cryoablation system failure ......................................................110