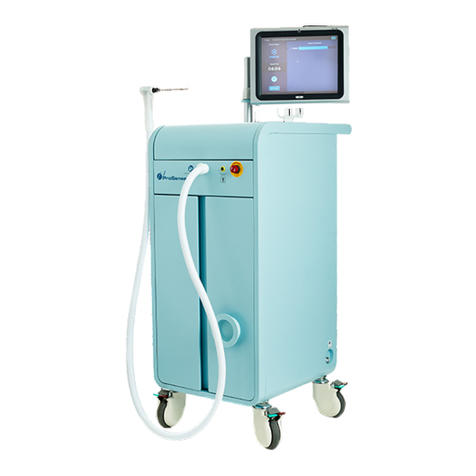
IceCure Medical Ltd. DSR3210000 rev. D ProSense™
Confidential User Manual
3
Return to first page
3.3.2 Emergency Stop button..................................................................................40
3.3.3 Flexible hose..................................................................................................41
3.3.4 Touch screen and User Manual activation.....................................................41
3.3.5 Cryohandle and cryoprobe.............................................................................43
3.3.6 Holder –Optional (Not available in some regions, e.g. China).....................45
3.3.7 Foot pedal (Not available in some regions, e.g. China).................................46
3.3.8 Temperature sensor (TS) ...............................................................................46
3.3.9 Dewar storage cases.......................................................................................48
3.4 Operational details ................................................................................................49
3.4.1 Starting the system.........................................................................................49
3.4.2 System pre-tests.............................................................................................50
3.4.3 Cryoablation procedure..................................................................................51
3.4.4 EXTRACTION step ......................................................................................51
4INSTALLATION AND SETUP .................................................................................52
4.1 Space and positioning requirements......................................................................52
4.2 Setup warnings and cautions.................................................................................52
4.3 Electrical requirements..........................................................................................53
4.3.1 For countries in which local line voltage is 100-127 VAC ...........................53
4.3.2 For countries in which local line voltage is 220-240 VAC ...........................53
4.4 Shipment components...........................................................................................53
4.5 Installation.............................................................................................................54
5OPERATING THE SYSTEM .....................................................................................55
5.1 Procedure Overview..............................................................................................55
5.2 Pre-operational stages ...........................................................................................55
5.2.1 Preparing the system for procedure ...............................................................55
5.2.2 Preparing the patient for procedure ...............................................................56
5.2.3 Switching on the ProSense™ cryoablation system .......................................57
5.2.4 Cryohandle.....................................................................................................58
5.2.5 General settings..............................................................................................58
5.2.6 Preparing the system for treatment................................................................62
5.2.7 Treatment Selection.......................................................................................79
5.3 Operational stages.................................................................................................84
5.3.1 Safe Operation in Percutaneous Procedures..................................................84
5.3.2 Preliminaries..................................................................................................84
5.3.3 Freeze Cycle ..................................................................................................87
5.3.4 Pause Option:.................................................................................................87
5.3.5 THAW ...........................................................................................................92
5.3.6 EXTRACTION process.................................................................................92
5.3.7 Show Last Treatment.....................................................................................97
5.3.8 Replace (refill) the Dewar during a cryoprocedure .......................................99
5.4 Post-operational stages........................................................................................104
5.4.1 Removing the temperature sensor from tissue.............................................104
5.4.2 Removing the cryoprobe from the cryohandle ............................................104
5.4.3 Disassembling the support device - Hose-Holder........................................105
5.4.4 Disassembling the temperature sensor.........................................................105
5.4.5 Exiting the ProSense™ cryoablation system treatment mode.....................105
5.5 Instruct patients:..................................................................................................106
5.6 System failures....................................................................................................107