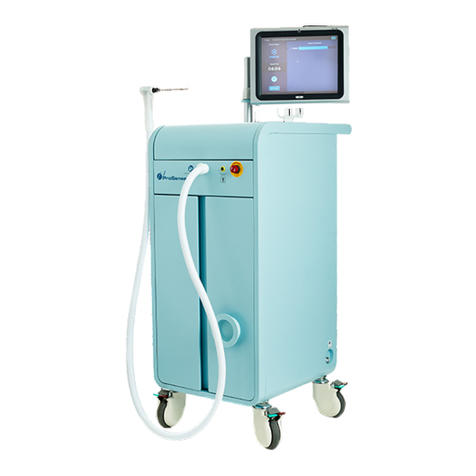
IceCure™ Medical Ltd. DMS-7064 rev. C ProSense™
Confidential User Manual
6
European User Manual
Figure 41 : Schematic illustration of placement of the system and patient. ......................57
Figure 42: System is loading screen.....................................................................................58
Figure 43: Main Menu screen..............................................................................................59
Figure 44: Action button as displayed on screen (several of the options)..........................59
Figure 45: Extraction button as displayed on screen ..........................................................59
Figure 46: Activating the Settings option from the Main Menu screen..............................60
Figure 47: Settings screen....................................................................................................60
Figure 48: Language Selection screen .................................................................................61
Figure 49:Technician Mode screen......................................................................................61
Figure 50: Export Log screen................................................................................................62
Figure 51: Windows® Date and Time Properties screen .....................................................63
Figure 52: Preparing the system for treatment by pressing Prepare for Treatment on Main
Menu screen ........................................................................................................................64
Figure 53: Dewar preparation screen..................................................................................64
Figure 54: Frost on the Dewar opening ...............................................................................65
Figure 55: Freezing Has Stopped Pop-up Message .............................................................65
Figure 56: Correct insertion and positioning of the Dewar –bottom first..........................67
Figure 57: Insertion of the Dewar –head first, leaning forward.........................................67
Figure 58: Insertion of the Dewar - bottom first, leaning backwards ................................67
Figure 59: Dewar door open warning..................................................................................68
Figure 60: Ice ball measurement ranges .............................................................................69
Figure 61: Cryoprobe (the figure is for illustration only).....................................................71
Figure 62: Cryoprobe registration screen............................................................................72
Figure 63: Invalid serial number warning ............................................................................72
Figure 64 : “PEEL HERE” label ..............................................................................................73
Figure 65: Cryoprobe connection screen.............................................................................74
Figure 66: Wait for Functional test screen ..........................................................................77
Figure 67: Functional test screen.........................................................................................77
Figure 68: Functional test - visual inspection screens.........................................................78
Figure 69: Pre-Planning trajectory needle insertion in breast applications........................80
Figure 70: Select Organ........................................................................................................80
Figure 71: Lesion size value .................................................................................................81
Figure 72: Planned Margin...................................................................................................81
Figure 73: Calculated Result N/A .........................................................................................82
Figure 74: Treatment Selection screen................................................................................82
Figure 75: Edit Presets in the Main Menu screen................................................................83
Figure 76: Define a treatment protocol by choosing Setup Presets on the Treatment
Selection screen...................................................................................................................83
Figure 77: Steps for adding a protocol ...............................................................................85
Figure 78: Edit Preset screen ...............................................................................................85
Figure 79: Choosing the Manual Mode option....................................................................86
Figure 80: Introducer shaft length.......................................................................................88
Figure 81: Illustration of FAP7200000 cryoprobe Markings on the cryoprobe...................88
Figure 82: Left: Pause button available; Right: Pause in progress, Play button available ..90
Figure 83: Manual Mode screen: press on Freeze to start the Freeze step........................90
Figure 84: Freeze screen in Manual Mode ..........................................................................91