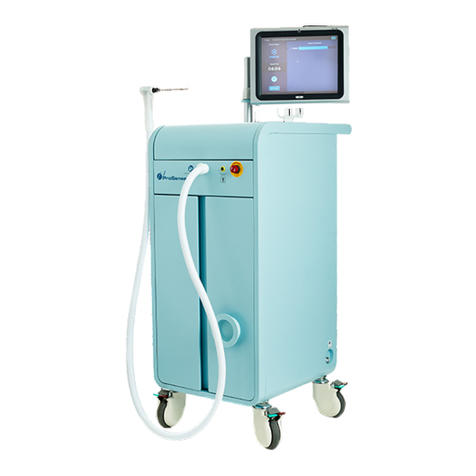
temperature sensor, cryohandle, flexible hose and touch screen
covers and sleeves).
All single use devices are considered medical waste and must be
disposed off in accordance with medical waste laws and hospital
standards. Sharp objects such as the cryoprobe and temperature
sensor must be disposed of in a sharps container.
The cryoprobes are single use and are suppliedin single
use packaging. Never reuse a single-use cryoprobe.
Reprocessing single use device (like the cryoprobe, introducer and
temperature sensor) could affect the mechanical or performance
or microbiological properties of the product.
Contraindications
There are no known contraindications specific to the use of
IceCure’s cryoablation systems and its’ Cryoprobes.
Potential Adverse Events
Frostbite are known adverse events related to the specific use of
the Cryoablation cryoprobes. There are, however, potential
adverse events associated with any surgical procedure. Potential
adverse events which may be associated with the use of
cryoablation may be organ specific or general and may include, but
are not limited to myocardial infraction, abscess, adjacent organ
injury, allergic/anaphylactoid reaction, angina/coronary ischemia,
arrhythmia, atelectasis, bladder neck contracture, bladder spasms,
bleeding/hemorrhage, creation of false urethral passage,
creatinine elevation, cystitis, diarrhea, death, delayed/non healing,
disseminated intravascular coagulation (DIC), deep vein thrombosis
(DVT), ecchymosis, edema/swelling, ejaculatory dysfunction,
erectile dysfunction (organic impotence), fever, fistula, hematoma,
hematuria, hypertension, hypotension, hypothermia, idiosyncratic
reaction, ileus, impotence, infection and thermal injury to the skin,
injection site reaction, myocardial infarction, nausea, neuropathy,
obstruction, organ failure, pain, pelvic pain, pelvic vein thrombosis,
penile tingling/numbness, perirenal fluid collection, mild vaginal
staining, pneumonia, empyema, hemoptysis, lung collapse,
thrombosis (evolved risk for patient with an existing AV fistula),
phrenic nerve palsy, cough, back pain, skin injury, pulmonary
emboli, Loss of speech (temporary aphasia from recurrent
laryngeal nerve damage, arm paresis, burn, hemorrhage,
pneumonitis, Respiratory failure/arrest, Hemothorax, pneumonia,
empyema, lung collapse, thrombosis (evolved Risk for pt. with an
existing AV fistula), phrenic nerve palsy, subcutaneous
emphysema, death due to Acute respiratory distress syndrome,
pulmonary emboli, tumor recurrence, hemorrhage, dyspnea,
hematoma pleural effusion, pneumothorax, cryoprobe site
paresthesia, prolonged chest tube drainage, prolonged intubation,
pulmonary embolism, pulmonary insufficiency / failure, rectal pain,
renal fracture, renal infract, genitourinary perforation, glomerular
filtration rate elevation, transfusion, infection lumbar
radiculopathy, transfusion, acute bowel injury, colorenal fistula,
exuberant local disease, infection lumbar radiculopathy,
myocardial infraction, transfusion, acute bowel injury, colorenal
fistula, exuberant local disease, renal artery/renal vein injury, renal
capsule fracture, renal failure, renal hemorrhage, renal infarct,
renal obstruction, renal vein thrombosis, rectourethral fistula,
scrotal edema, sepsis, skin burn, stricture of the collection system
or ureters, stroke, thrombosis/thrombus/embolism, transient
ischemic attack, tumor seeding, UPJ obstruction/injury, urethral
sloughing, urethral stricture, urinary fistula, urinary frequency/
urgency, urinary incontinence, urinary leak, urinary renal leakage,
urinary retention/ oliguria, urinary tract infection, vagal reaction,
voiding complication including irritative voiding symptoms,
vomiting and wound complication. muscular injury, pain, swelling,
Osteonecrosis, osteomyelitis, Chondrolysis (rapid and sudden
damage to the cartilage results in arthritis), damage to
Osteocartilage structure, Nerve Palsy, Motor Dysfunction, Peri-
ablational neuropathies, swelling up to compartment syndrome,
bowel damage, urinary tract damage, pericardial effusion in (in
chest wall ablations), avascular necrosis of femur head, ureteral
stricture, tumor recurrence.
Cryoprobe technical specifications
The packed cryoprobes shall be stored in a dry, cool, well-ventilated
and clean environment without corrosive gas.
In general, IceCure's Cryoprobes are available in various diameters
(2.4mmto 3.4mm), variousice ball shapes (Spheric, Ellipsoid), various
tips (trocarand pencil) and various lengths (127mm to 185mm
external shaft length) according to the expected application, treated
tumor size and surgery approach.
The opening of the cryoprobe pouch should be
where the “PEEL HERE” label is positioned.
Figure 6: “Peel Here” label
Ref number: FAP 7100000, FAP 7200000, FAP 7400000,
FAP 7410000, FAP 7600000 and FAP 7800000.
Range temperature: -196C to +40C
Needle diameter: 2.4mm (13G) or 3.4mm (10G)
Certain configurations are not available in some regions.
IceCure Medical, Inc.
41-18 Christine Ct
Fair Lawn, NJ, 07410, USA
icecuresupport@icecure-medical.com
Tel: +1 (646) 8443066
IceCure Medical Ltd.
7 Haeshel St., 2nd floor,
Caesarea 3079504, Israel
info@icecure-medical.com
Tel: +972-4-623 0333; Fax: +972-4-623 0222