5
> DEVICE DESCRIPTION
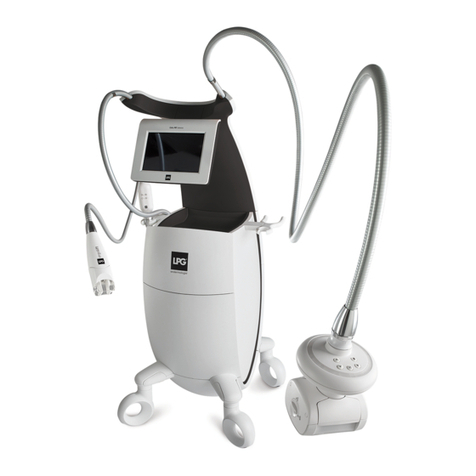
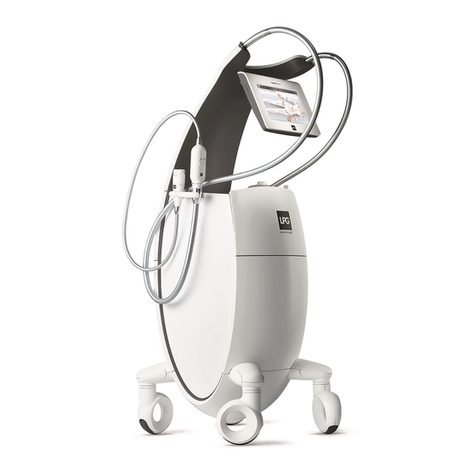
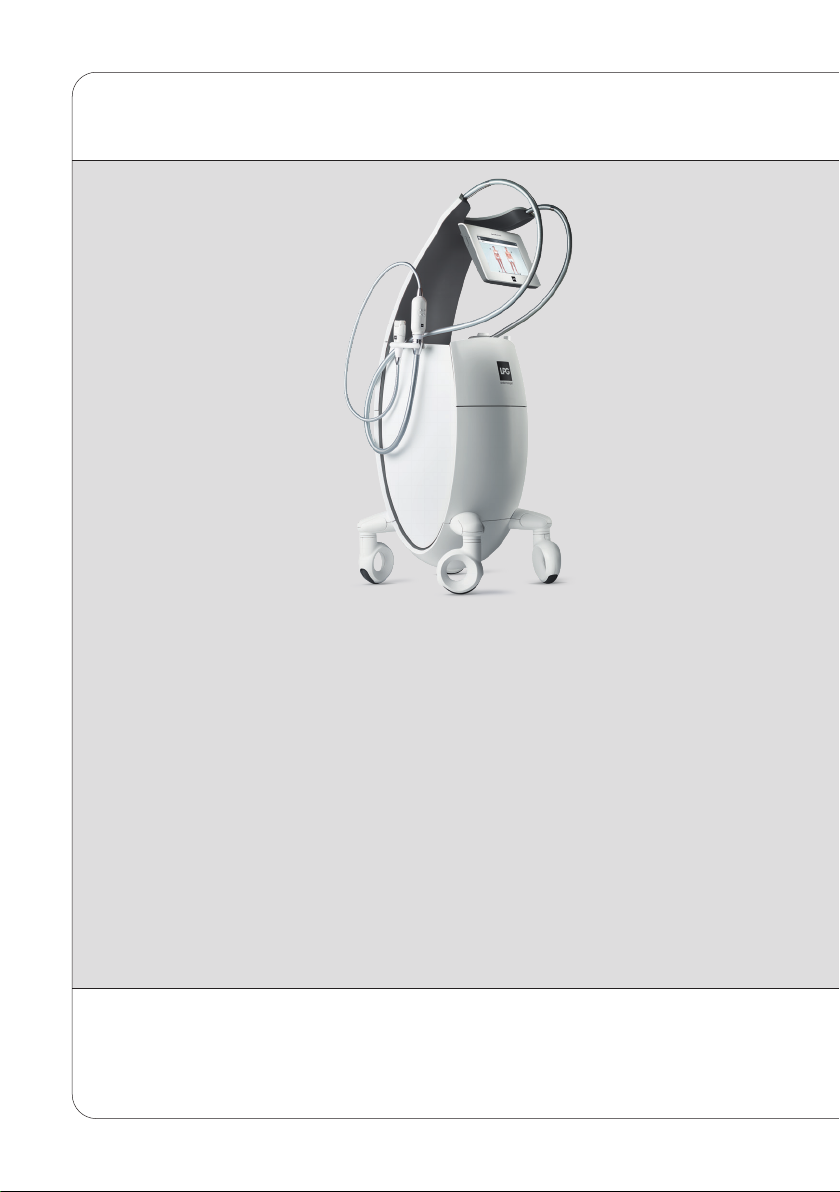
DEVICE DESCRIPTION
1
INTENTED USE
The CELU M6 Alliance®Medical device is intended for therapeutic use. It can be used to:
1. Reduce secondary lymphedema of the arm after a mastectomy
2. Improve secondary lymphedema
3. Improve lymphatic circulation in the treated area
4. Relieve minor muscle aches and pains
5. Relieve muscle spasms
6. Temporarily improve local blood circulation
7. Temporarily relieve minor muscular pain associated with DOMS
(Delayed Onset Muscle Soreness)
8. Improve local circulation during burn rehabilitation
9. Reduce the appearance of cellulite and the circumference of treated areas
10. Temporarily improve lymphatic circulation and local blood circulation to improve skin
trophicity in the treated areas
11. Improve skin quality, scars, fibrosis
12. Improve skin aging (wrinkles, fine lines, skin sagging, fat infiltration, firmness,
elasticity, complexion and eye bags)
13. Stimulatie fibroblasts (collagen, elastin and hyaluronic acid synthesis)
The device can be used in hospitals, therapy centers and institutions by specialists and
physiotherapists. It can be used on adult patients only, of any weight or any sex. It is an
independent device that cannot be combined with other machines. It is to be used by
professionals who are specially trained by LPG Systems in the use of the device and is
not suitable for home use.
OPERATING PRINCIPLES:
The operating principles of the CELLU M6 Alliance®Medical device consist of a suction
force coupled with movements of rolls/valves, performed with treatment heads. These
heads are placed on the healthy skin of the patient and then moved across the area to be
treated by the professional who has been trained by LPG Systems.