iv
NOTE
This Operations Manual will describe both the MagnitudeTM 3150M and Omni-TrakTM 3150 MRI
Monitors in detail. For simplicity the manual will refer to both these systems by the name “3150(M) MRI
Monitor”except in the cases where a feature applies only to one of the two systems, in which case the
specific 3150(M) MRI system will be referenced.
PRECAUTIONS
General
Federal law in the USA or Canada restricts this device to sale by, or on , the order of a physician.
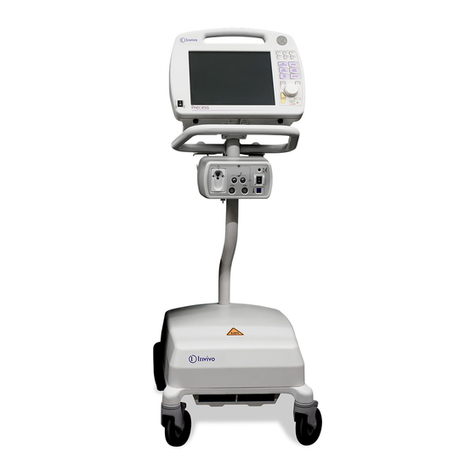
The 3150(M) MRI Patient Monitoring System is comprised of the 3150(M) MRI Monitor and a
Remote Monitor (Millennia®3155MVS or 3155A). Always operate the 3150(M) MRI Monitor with
its designated remote monitor.
The accuracy of the measurements can be affected by the position of the patient, the patient’s
physiological condition, and other factors. Always consult a physician for interpretation of
measurements made by this monitor.
To avoid remote monitor fall, secure monitor on the shelf or bracket prior to use.
An explosion hazard exists if this monitor is used in the presence of flammable anesthetics.
The operator should read and thoroughly understand this manual and the associated remote monitor
manual (Invivo Research, Inc. Part Number 9545) completely before attempting to operate the 3150(M)
MRI Monitor.
If any system failure occurs (e.g. an unexplained continuous audible alarm) remove the monitor from
use, and refer it to qualified service personnel.
When the message “SOUND OFF”is displayed on the 3150(M) MRI Monitor (an “X” is displayed on
the screen of the remote 3155 MVS or 3155A monitor), the audible alarm tone will not sound for any
reason.
Perform operational checkout before each use. If monitor fails to function properly, refer to qualified
service personnel.
For safe and accurate operation and MRI compatibility, use only recommended Invivo Research patient
electrodes, cabling, lead wires, cuffs, hoses, sensors, tubing, etc. A listing of these can be found in the
Accessory Listing within this manual, or by contacting Invivo Research directly.
For continued operation, always connect the monitor to AC Main Power through the AS153 AC Power
Adapter when a Low Battery indication occurs. Failure to do this can lead to interruption of monitoring
and/or damage to the monitor’s battery(s).
The system may not conform to all performance specifications if stored or used outside the
environmental specifications identified in Appendix A in the rear of this manual.
Do not apply any unnecessary pressure to the screen area of the monitor. Severe pressure applied to this
portion of the monitor could result in damage or failure of this screen.
All equipment not complying with IEC 601-1 should be placed outside the patient environment. Only
connect IEC 601-1 compliant equipment to this monitor. To avoid potentially hazardous leakage
currents, always check the summation of leakage currents when several items of equipment are
interconnected.
For proper equipment maintenance, perform the service procedures at the recommended intervals as
described in the monitor’s service manual.
Single use devices should never be reused.