i
TABLE OF CONTENTS
Paragraph Number Page Number
List of Figures ........................................................................................................................................... iv
List of Tables.............................................................................................................................................. v
Equipment Classification ........................................................................................................................ v
Precautions ................................................................................................................................................ vi
User Responsibility ................................................................................................................................xiv
3160 MRI Physiological Monitor Accessories.................................................................................. xv
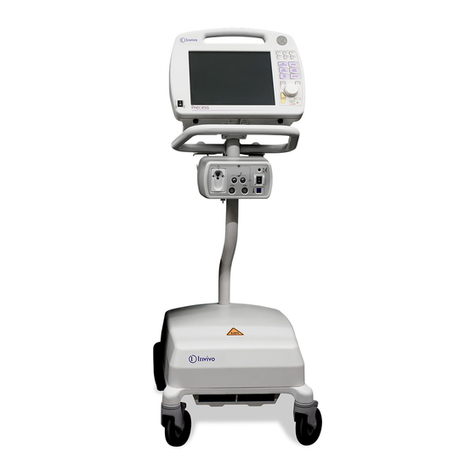
1.0 INTRODUCTION ......................................................................................................... 1-1
1.1 Product Description ......................................................................................................... 1-1
1.1.1 System Mounting................................................................................................. 1-1
1.1.2 System Parameters............................................................................................... 1-1
1.1.3 User Interface....................................................................................................... 1-2
1.1.4 Versatility............................................................................................................. 1-2
1.2 Wireless Processor Unit (WPU) ...................................................................................... 1-2
1.2.1 Operating Environment........................................................................................ 1-2
1.2.2 Power Supply....................................................................................................... 1-2
1.2.3 Battery Operation................................................................................................. 1-2
1.3 Patient Connections ......................................................................................................... 1-2
1.3.1 NIBP and Agent Monitoring................................................................................ 1-2
1.3.2 ECG Monitoring .................................................................................................. 1-3
1.3.3 SpO2 Monitoring ................................................................................................. 1-3
1.4 Display Control Unit (DCU)............................................................................................ 1-3
1.4.1 DCU Controls ...................................................................................................... 1-3
1.4.2 DCU Display........................................................................................................ 1-8
1.5 Cleaning ......................................................................................................................... 1-12
1.5.1 Cleaning Accessories......................................................................................... 1-12
2.0 INSTALLATION........................................................................................................... 2-1
2.1 Introduction...................................................................................................................... 2-1
2.2 Monitor Installation ......................................................................................................... 2-1
2.2.1 Monitor Mounting................................................................................................ 2-1
2.2.2 Preparing the 3160 MRI Physiological Monitoring System for Use................... 2-1
2.2.3 Monitor Start Up.................................................................................................. 2-1
3.0 PREPARATION FOR USE.......................................................................................... 3-1
3.1 Introduction...................................................................................................................... 3-1
3.2 SETUPS Menu................................................................................................................. 3-1
3.3 Store/Recall Setups ........................................................................................................ 3-20
3.4 Monitor Initialization..................................................................................................... 3-21
3.4.1 Default Initialization .......................................................................................... 3-21
3.4.2 Pre-Configured Initialization ............................................................................. 3-21
4.0 PATIENT PARAMETERS........................................................................................... 4-1
4.1 ECG Monitoring .............................................................................................................. 4-1
4.1.1 Patient and Lead Preparation ............................................................................... 4-1
4.1.2 Associated Waveforms and Displays .................................................................. 4-2
4.1.3 The ECG Menu.................................................................................................... 4-2
4.1.4 Alarm Limits........................................................................................................ 4-5
4.1.5 Trended Data........................................................................................................ 4-5
4.1.6 ECG Messages..................................................................................................... 4-5