7/ 21
4. The product shall not be used in combination with MRI or CT equipment.
5. Do not squeeze the silica gel pad with your fingers during use.
6. The product shall not be used in flammable and explosive environment.
7. The product plays a supporting role in the diagnosis of patients only. A diagnosis should be made based on clinical
manifestations and symptoms.
8. Test point should be periodically changed during long-term use or depending on patient's conditions. Test point
should be changed, and patient's skin integrity and circulation condition should be checked to make appropriate
adjustments at least every 2 hours.
9. Autoclaving, vinyl oxide disinfectant, or immersing the sensor in liquid disinfectant can cause erroneous
readings.
10. The device specified in this manual, reusable accessories or parts of accessories including batteries should
comply with local laws and regulations.
11.The device complies with the electromagnetic compatibility requirements for electronic medical products or
systems in IEC60601-1-2. Radio transmission equipment or other electromagnetic interference may affect the
performance of this device.
12. Portable radio communication equipment may affect the performance of this device.
13. The device should not be used in the vicinity of other equipment or stacked on other equipment
14. Use of the device is not recommended during transportation of patients, such as on an ambulance.
15. Do not disassemble, install or repair the device without authorization.
16. The materials in contact with the patient is medical silica gelpad,The material meet the requirements of ISO
10993.
17.Temperature shall not exceed 40℃when in contact with human body. The recommended maximum
application time are 1-2 hours.
18. The device is not indicated for children weighing less than 20 kg, pregnant women or nursing mothers.
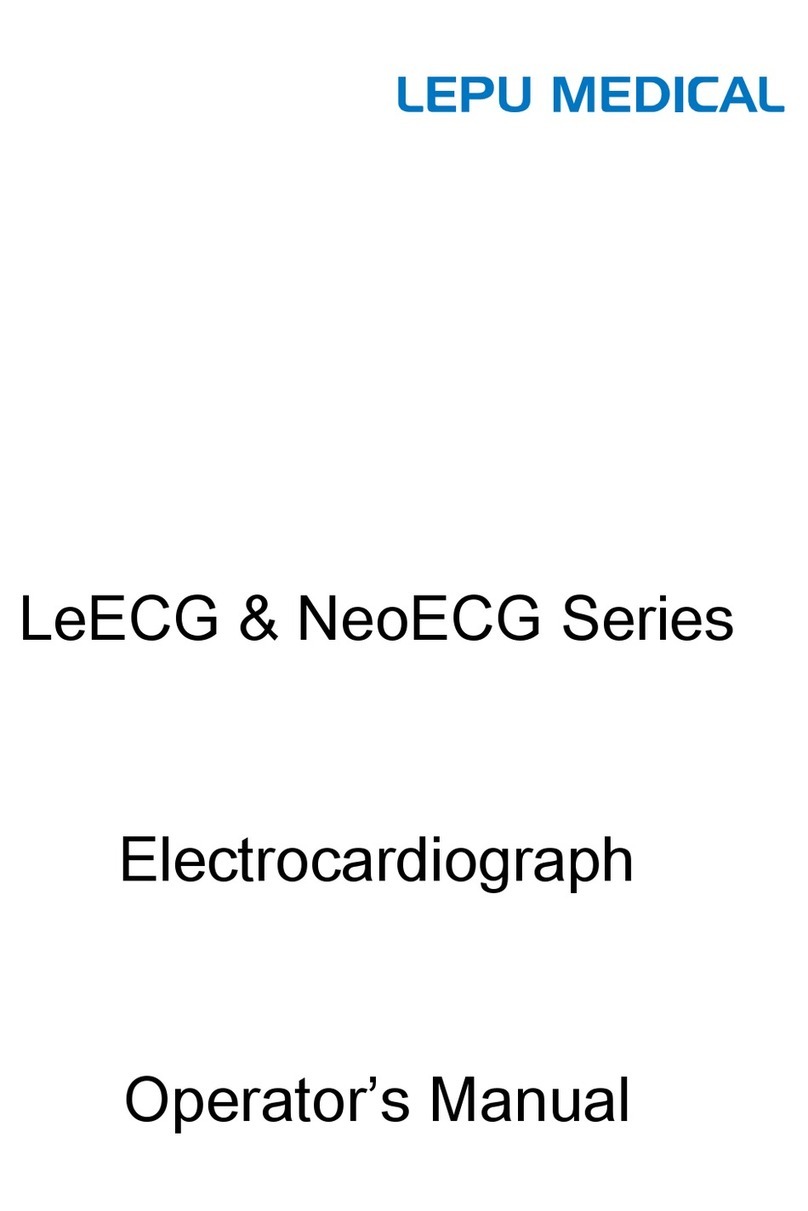
19.Do not dispose of batteries in fire.Dispose of batteries in accordance with the local ordinances and
regulations.