EN EN
Service life 5 years
Useful life Up to 12 months1
Therapy pressure 4 hPa - 30 hPa
Quoted two-figure noise emis-
sion value according to ISO
4871:
Sound pressure level
Sound power level
Uncertainty factor
18 dB(A)
26 dB(A)
3 dB(A)
Temperature:
Operation
Transport and storage
+5 °C to + 40 °C
-20 °C to +70 °C
Standards applied EN ISO 17510: 2020
Product class to MDR (EU)
2017/745
IIa
1 Useful life depends on cleaning and on the deter-
gents used, on the amount of time worn daily, on
therapy pressure, and on individual secretion of
sweat. Using the second mask cushion (included as
an option) does not extend useful life.
8 Materials
No parts of the mask contain latex, PVC (polyvinyl
chloride) or DEHP (diethylhexyl phthalate). In the
event of allergies to the materials listed, only use the
mask following agreement with the healthcare pro-
fessional.
Headgear CO (cotton), EL (elastane), P
(polyester), PU
(polyurethane)
Headgear clip PA (polyamide)
Forehead support PA (polyamide)
Mask body PA (polyamide)
Mask cushion SI (silicone)
Elbow PA (polyamide)
Rotating sleeve PA (polyamide)
9 Characteristic pressure/flow
curve
0
10
20
30
40
50
60
0 5 10 15 20 25 30
Therapy pressure (hPa)
Mean intentional leakage (l/min)
10 Markings and symbols
The following markings and symbols may be applied
to the device, accessories or packaging.
Symbol Description
Unique device identifier (uniform product
code for medical devices)
Order number
Indicates the product is a medical device
Lot number
Manufacturer and, if necessary, date of
manufacture
Follow the instructions for use
CE symbol (confirms that the product
conforms to the applicable European di-
rectives/regulations)
Permitted temperature range for trans-
port and storage
Use by date
Keep out of sunlight
11 Warranty
Löwenstein Medical Technology gives the purchaser
of a new original Löwenstein Medical Technology
product and of a spare part fitted by Löwenstein
Medical Technology a limited manufacturer warranty
in accordance with the warranty conditions applica-
ble to the product in question and in accordance
with the warranty periods from date of purchase
listed below. The warranty conditions are available
on the manufacturer’s website. We will also send you
the warranty conditions on request.
Please bear in mind that any claim under warranty
and liability shall be void if neither the accessories
recommended in the instructions for use nor gen-
uine spare parts are used.
In the event of a claim under warranty, contact your
specialist dealer.
Product Warranty periods
Masks including accessories 6 months
12 Declaration of Conformity
The manufacturer Löwenstein Medical Technology
GmbH + Co. KG (Kronsaalsweg 40, 22525 Hamburg,
Germany) hereby declares that the product complies
with the relevant provisions of the Medical Device
Regulations (EU) 2017/745. The unabridged text of
the Declaration of Conformity can be found on the
manufacturer’s website.
In the EU: As a user and/or patient, you must report
any serious incidents occurring in conjunction with
the product to the manufacturer and to the responsi-
ble authority.
1 Operation
The figures show the following steps for operating
the mask:
Putting on the mask
Adjusting the mask
Removing the mask
Dismantling the mask
Assembling the mask
For blind and partially-sighted users
An electronic version of the instructions for use
is also available on the manufacturer’s website.
2 Introduction
2.1 Intended use
The CARA mask is used for treating sleep apnea and
for non-invasive and non-life-sustaining ventilation
of patients with respiratory insufficiency. It serves as
a link between the patient and the ventilator.
2.2 Contraindications
The mask must not be used on patients weighing <
30 kg.
The mask must not be used in the following situa-
tions: Immediate intubation required, loss of con-
sciousness, acute vomiting.
The mask may be used in the following situations
only with particular caution: Pressure points and
acute injuries to the skin of the face; skin allergies in-
volving the face; deformities of the face or nasophar-
ynx; acute pain affecting the face; cough reflex re-
stricted or absent; claustrophobia; acute nausea.
If you are not sure whether one of these situations
applies to you, consult your healthcare professional.
Observe the contraindications in the instructions for
use of your device.
2.3 Side effects
The following side effects may occur with use of the
mask: Nasal congestion, dry nose, dry mouth in the
morning, feeling of pressure in the sinuses, irritated
conjunctiva, skin rashes, pressure marks on the face,
irritating noises when breathing.
If these side effects occur, contact your healthcare
professional.
2.4 Clinical benefit
Transfers the therapeutic efficacy of the ventilator to
the patient
3 Safety
Risk of injury due to damaged mask parts or
those under strain!
⇒
Perform a visual inspection before every use and
after every cleaning operation.
⇒
Note useful life (see section entitled “Technical
Data”).
⇒
Replace mask parts if necessary.
Risk of injury due to the use of oxygen!
Oxygen can become deposited in clothing, bedlinen,
and hair. Supplying oxygen without a safety device
can lead to fire.
⇒
Use an oxygen safety valve.
⇒
Follow the instructions for use for the oxygen
supply system.
⇒
Set up oxygen sources at a distance > 1 m from
the device.
⇒
Do not smoke.
⇒
Avoid naked flames.
⇒
Ventilate the room well.
⇒
Keep mask free from oil and grease.
Risk of injury due to patient receiving inade-
quate supply!
⇒
Activate low pressure/leakage alarms on the
ventilator.
⇒
Use the appropriate mask size and check that it
is securely in position.
⇒
Monitor patients with restricted spontaneous
breathing.
Risk of injury from re-inhalation of CO2!
⇒
Only use the mask when therapy is in progress.
⇒
Only use the mask within the quoted therapy
pressure range.
⇒
Patients unable to remove the mask themselves
must be monitored by a nurse.
⇒
Do not close off exhalation systems.
Risk of injury due to escape of anesthetic gas
or atomization of drugs!
⇒
Do not use the mask during anesthesia.
⇒
Do not use the mask to atomize drugs.
Risk of injury from inadequate cleaning!
⇒
Clean mask parts before using for the first time
(see section entitled "Cleaning and reprocess-
ing").
⇒
Clean the mask regularly.
⇒
When selecting a detergent, consider potential
allergies.
⇒
On change of patient in a hospital environment:
Comply with the document entitled
Information
on reprocessing
(see section entitled “Repro-
cessing”).
⇒
For patients with a compromised immune sys-
tem or particular background of illness, disinfect
mask parts daily following consultation with the
healthcare professional.
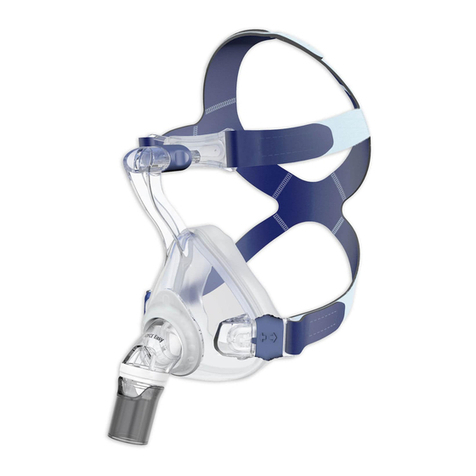
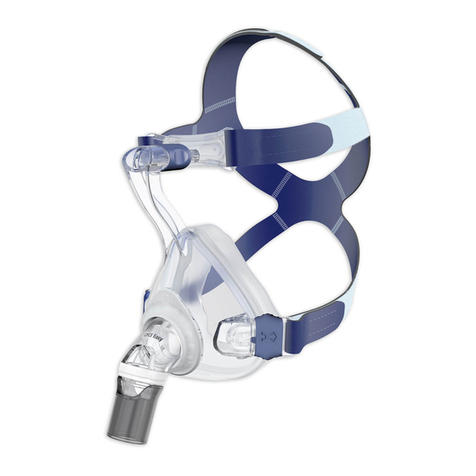
4 Product description
A diagram of the individual parts can be found on
the title page.
1 Headgear 4 Rotating sleeve
2 Forehead support 5 Mask body
3 Elbow 6 Mask cushion
Compatible devices
In some device combinations, actual pressure does
not correspond to the therapy pressure displayed by
the device. Have the device adjusted by a healthcare
professional so that actual pressure in the mask cor-
responds to therapy pressure. This setting should be
made using the type of mask used during therapy.
Exhalation system
The mask has an integrated exhalation system. The
exhaled air escapes through a gap.
5 Cleaning and reprocessing
5.1 Clean mask
1.
Wash your hands before starting cleaning.
2.
Dismantle mask (see Figure ).
3.
Wash mask by hand (max. 30 °C, 1 ml mild deter-
gent in 1 l water) in accordance with the table be-
low:
Mask part Frequency Action
All mask
parts
Daily Soak and wash for 15
minutes and clean for 3
minutes using a soft
cleaning brush.
Headgear Weekly Wash for 15 minutes.
All parts can be washed in a dishwasher once a
week (max. 70 °C, mild detergent, max. pro-
gram length 90 minutes, top basket, separate
rinse).
4.
Rinse all parts with clean water.
5.
Allow all parts to air-dry.
6.
Perform a visual inspection for cracks and defor-
mation. Replace damaged parts. Discoloration is
not a problem.
7.
Assemble mask (see Figure ).
5.2 Reprocessing (clinical environment)
In the event of a change of patient, follow the in-
structions in the document entitled
Information on
reprocessing
. The document can be found on the
manufacturer’s website. We will send you the docu-
ment on request.
5.3 Disposal
Dispose of the mask in domestic waste. In the clinical
environment: Dispose of the mask in accordance
with hospital regulations.
6 Troubleshooting
Fault Cause Action
Pain due to
pressure on
the face
Mask too
tight.
Loosen headgear.
Draft in the
eye
Mask too
loose.
Tighten headgear.
Mask does not
fit.
Contact your spe-
cialist dealer.
Therapy pres-
sure not
reached.
Mask not cor-
rectly ad-
justed.
Re-adjust mask.
Mask cushion
damaged.
Replace mask
cushion.
Patient circuit
damaged.
Check circuit and
correct fit of cir-
cuit.
7 Technical data
Dimensions in mm
(W x H x D)
Size XS
Size S/M
Size M/L
72 x 112 x 70
72 x 117 x 70
72 x 118 x 70
Weight
Size XS
Size S/M
Size M/L
58 g
59 g
60 g
Dead space
Size XS
Size S/M
Size M/L
74 ml
76 ml
79 ml
Tube connection: Tapered
connection to EN ISO 5356-1
Ø 22 mm (male)
Flow resistance
at 50 l/min
at 100 l/min
0.2 hPa
0.9 hPa
WM 68325b 09/2023 ZH-CHT, EN-US