Manamed ManaSport+ ManaFlow 51 User manual
INSTRUCTIONS FOR USE
Noninvasive
UItrasound Device
Customer Service
Toll Free: 888-508-0712
Email: CustomerService@manamed.com
Web: www.manamed.com
Manufactured For:
5240 W Charleston Blvd., #150,
Las Vegas NV 89146
Scan QR Code to watch
Instructions for Use
TD-MSPORT02-IFU, Rev. 01
Copyright 2023 ManaMed®, LLC

Table of Contents
Purpose of Device . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 3
Indications for Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 3
Contraindications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 3
Warnings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Pages 3 - 4
Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 4
Be Aware of the Following . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 4
Adverse Reactions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 4
Quick Start Guide . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Pages 5 - 7
Optional Smart Device App Guide . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Pages 8 - 10
User Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 11
Storage . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 11
Disposal . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 11
Using the AC Adapter / Battery Charger . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 11
Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 11
Ultrasound Gel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 12
ManaSport+ Expected Service/Shelf Life . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 12
Limited Warranty . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 12
Technical . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 12
Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 13
Instructions for reporting adverse events . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 13
Summary of ManaSport+ Wireless Functions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 13
Summary of ManaSport+ Wireless Security Methods . . . . . . . . . . . . . . . . . . . . . . . . Page 13
Radio Frequency Wireless Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 13
Radio Frequency Wireless Description . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 14
Electromagnetic Compatibility Tables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Pages 15 - 16
Standards . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 17
FCC Statement . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 17
PURPOSE OF DEVICE
The ManaSport+ is a portable and rechargeable prescriptive device.
It is intended to be used for adults only, under the direction of a medical professional. The ManaSport+ is intended for Home Use.
INDICATIONS FOR USE
CONTRAINDICATIONS
Do not use this device on persons whose pain syndromes are undiagnosed. Contraindications for the use of ultrasound include:
t0WFSBOBSFBPGUIFCPEZXIFSFBNBMJHOBODZJTLOPXOUPCFQSFTFOU
t0WFSUIFFZFT
t0WFSPSOFBSHSPXUIDFOUFSTVOUJMCPOFHSPXUIJTDPNQMFUF
t0WFSUIFSFQSPEVDUJWFPSHBOT
t0WFSUIFQSFHOBOUVUFSVT
t0WFSBIFBMJOHCPOFGSBDUVSF
t0WFSBOBDUJWFJNQMBOUFENFEJDBMEFWJDFTVDIBTBOJNQMBOUFEEFFQCSBJOTUJNVMBUJPOEFWJDF
t0OUIFCSBJOTQJOBMDPSEPSMBSHFTVCDVUBOFPVTQFSJQIFSBMOFSWFT
t
Ischemic tissues in individuals with vascular disease where the blood supply would be unable to follow the increase in metabolic demand and tissue necrosis might result
t
Ultrasound therapy should not be used for symptomatic local pain relief unless etiology is established or unless a pain syndrome has been diagnosed
tApplication over parenchymatous organs – liver, spleen, lungs, endocrine glands, gonads
t#POFQSPUVCFSBODFTKVTUVOEFSUIFTLJOWFSUFCSBMTQJOPVTQSPDFTTFTBOLMFTFQJDPOEZMFT
t1FSJQIFSBMOFSWFTDMPTFVOEFSUIFTLJOTVSGBDF
tAllergies to the applied ultrasound gels
t/FBSCSBJODFSWJDBMHBOHMJBTQJOFMBNJOFDUPNZTJUFTDBODBVTFTQJOBMDPSEIFBUJOH
t5PUBMIJQBSUISPQMBTUJFTXJUINFUIZMNFUIBDSZMBUFPSIJHIEFOTJUZQPMZFUIZMFOF5IFTFIBWFBIJHIDPFóDJFOUPGBCTPSQUJPONPSFUIBOTPGUUJTTVF
and the prosthesis could loosen due to unstable cavitation in the cement
t
Arthroplasties—the eect on bony ingrowth arthroplasties is not well dened; for this reason the most prudent course is avoiding ultrasonic therapy over these areas
tIn an area of the body where infectious disease is present
tBlood vessels in poor condition should not be treated as the vessel walls could rupture as a result of the treatment
t
Patients suering from cardiac disease should not receive treatment over the cervical ganglia, the stellate ganglion, the thorax in the region of the heart,
PSUIFWBHVTOFSWFBTBSFøFYDPSPOBSZWBTPTQBTNNJHIUSFTVMU0OMZMPXJOUFOTJUJFTBOETIPSUUSFBUNFOUUJNFTTIPVMECFVTFEJGUIFTFQBUJFOUTBSFUSFBUFE
JOPUIFSBSFBTCFDBVTFUIFTUJNVMBUJPOPGQSBDUJDBMMZBOZBòFSFOUBVUPOPNJDOFSWFFTQFDJBMMZUIFWBHVTOFSWFJOUIFCPEZDPVMEDBVTFBDIBOHFJODBSEJBDSBUF
tPatients with thrombophlebitis or other potentially thromboembolic diseases should not be treated because a partially disintegrated clot could
result in an obstruction of the arterial supply to the brain, heart or lungs
t0WFSBSFBTPGSFDFOUCMFFEJOHPSIFNPSSIBHF
t0WFSBSFBTPGBDUJWFUVCFSDVMPTJT
WARNINGS
t*GUIFUSFBUNFOUJTSFQPSUFEBTQBJOGVMPSUPPIPUBUBOZQPJOUEVSJOHUSFBUNFOUUVSOPòEFWJDFBOESFNPWFUIFEFWJDFGSPNUIFTLJO
t*OTUSVDUUIFQBUJFOUUPJOGPSNUIFQSBDUJUJPOFSJGUIFQBUJFOUGFFMTBOZQBJOPSCVSOJOHEVSJOHUSFBUNFOU
t*OTUSVDUUIFQBUJFOUIPXUPUVSOPòUIF%FWJDFBOESFNPWFUIF"QQMJDBUPSJGUIFQBUJFOUGFFMTBOZQBJOPSCVSOJOHEVSJOHUSFBUNFOU
t5IF%FWJDFTIPVMECFLFQUPVUPGUIFSFBDIPGDIJMESFO
t5IFEFWJDFBOEBDDFTTPSJFTBSFOPUTUFSJMF%0/05BQQMZUIJTEFWJDFBOEBDDFTTPSJFTPWFSBOPQFOXPVOEPSJOøBNFETLJO
t%0/05BQQMZEJSFDUMZPWFSBCPOFUIBUJTOFBSUIFTLJOTVSGBDF
(continued next page)
Apply stationary use of ultrasound to:
Generate deep heat within body tissues for the treatment of selected medical conditions such as the relief of pain, the relief of muscle spasms,
the treatment of joint contractures, and the local increase in circulation.
Apply continuous movement of ultrasound for:
1. Pain.
2. Pain relief, muscle spasms, and joint contractures.
3. Relief of pain, muscle spasms, and joint contractures that may be associated with:
t"EIFTJWFDBQTVMJUJT
t#VSTJUJTXJUITMJHIUDBMDJöDBUJPO
t.ZPTJUJT
t4PGUUJTTVFJOKVSJFTBOE
t4IPSUFOFEUFOEPOTEVFUPQBTUJOKVSJFTBOETDBSUJTTVFT
4. Relief of pain, muscle spasms, and joint contractures resulting from:
t$BQTVMBSUJHIUOFTTBOE
t$BQTVMBSTDBSSJOH
5. Localized increase in blood ow.
6. Increased range of motion of contracted joint using heat and stretch techniques.
3

tDo not use over sensitive skin areas or in the presence of poor circulation. The unattended use of this device by children or incapacitated
persons may be dangerous. To reduce the risk of buns, electric shock, and re, this device must be used in accordance with the instructions.
tDo not crush the device and its accessories.
tCarefully examine the device and its accessories, and do not use if they show any sign of deterioration.
tDo not tamper with this device and its accessories in any way. There are no user serviceable parts. If for any reason they do not function
satisfactorily, return to the authorized service center at address given.
t%POPUVTFBUUIFTBNFUJNFBTPUIFSUPQJDBMBOBMHFTJDT
tHandle ultrasound applicator with care. Inappropriate handling of the ultrasound applicator may adversely aect its characteristics.
tAn appropriate coupling medium should be used in order to ensure energy transmission to the tissue.
WARNINGS (continued)
Precautions
Precaution should be taken when using the device:
t0WFSBOBSFBPGUIFTQJOBMDPSEGPMMPXJOHBMBNJOFDUPNZJFXIFONBKPSDPWFSJOHUJTTVFTIBWFCFFOSFNPWFE
t0OQBUJFOUTXJUIIFNPSSIBHJDEJBUIFTFT
t0WFSBSFBTXIFSFNFUBMQSPTUIFTJTPSPUIFSNFUBMMJDJNQMBOUTBSFFNCFEEFEJOUJTTVFXIJDINBZGPSNB
reective surface to the ultrasound energy causing unintended irradiation of tissue and excessive heating.
t0WFSBOBDVUFJOGFDUJPOPSTFQTJT
t0OQBUJFOUTXJUIQFSJQIFSBMBSUFSZEJTFBTF
t0WFSBEFFQWFJOUISPNCPTJT
t0WFSBOBOFTUIFUJ[FEBSFBPSJODPOKVODUJPOXJUIBDPOEJUJPOUIBUDBVTFTJNQBJSNFOUPGTFOTBUJPOTVDIBTDBVTFECZDIFNPUIFSBQZ.
t$BVUJPOTIPVMECFVTFEGPSQFSTPOTXJUITVTQFDUFEPSEJBHOPTFEIFBSUQSPCMFNT
t$BVUJPOTIPVMECFVTFEGPSQFSTPOTXJUITVTQFDUFEPSEJBHOPTFEFQJMFQTZ
t$BVUJPOTIPVMECFVTFEJGZPVIBWFBOZPGUIFGPMMPXJOH
- iGZPVIBWFBUFOEFODZUPCMFFEJOUFSOBMMZGPMMPXJOHBOJOKVSZ
-JGZPVSFDFOUMZIBETVSHFSZPSIBWFFWFSIBETVSHFSZPOZPVSCBDL
- if areas of skin lack normal sensations, such as skin that is numb.
4
t$POTVMUXJUIZPVSQIZTJDJBOCFGPSFVTFPWFSUIFNFOTUSVBMVUFSVT
t
%POPUVTFUIJTEFWJDFXIJMFESJWJOHPQFSBUJOHNBDIJOFSZPSEVSJOHBOZBDUJWJUZJOXIJDIJOWPMVOUBSZNVTDMFDPOUSBDUJPOTNBZQVUUIFVTFSBUVOEVFSJTLPGJOKVSZ
t,FFQUIJTEFWJDFBOEJUTBDDFTTPSJFTPVUPGSFBDIPGDIJMESFOBOEQFUT*GTXBMMPXFEHFUNFEJDBMIFMQPSDPOUBDUB1PJTPO$POUSPM$FOUFSSJHIUBXBZ
t%POPUVTFUIJTEFWJDFJOIJHIIVNJEJUZBSFBTTVDIBTBCBUISPPN
t4UPQVTJOHUIJTEFWJDFBUPODFJGZPVGFFMEJTDPNGPSUEJ[[JOFTTPSOBVTFBBOEDPOTVMUZPVSQIZTJDJBO
t%POPUBUUFNQUUPNPWFUIFVMUSBTPVOEIFBEXIJMFUIFEFWJDFJTPQFSBUJOH
tFor ultrasound treatment, only use the ultrasound applicator sold with this device.
tWhen applying ultrasound by means of any applicator, ultrasound gel shall be used for correct passage of the ultrasound waves. It is
recommended to use an FDA cleared water-based conductive gel, specically 40/0.&%5&$)/0-0(:*/$(FM, The applicators
have not been tested for use with other gels or oils and can be damaged.
t0OQBUJFOUTXJUIIFNPSSIBHJDEJBUIFTFT
t0WFSBSFBTXIFSFUIFSFJTTFOTPSZJNQBJSNFOUPSTFOTPSZMPTT
t0WFSBDVUFTLJODPOEJUJPOTTVDIBTFD[FNBEFSNBUJUJTFUD
t0WFSUIFBOUFSJPSBTQFDUPGUIFOFDL
t0OQBUJFOUTXIPBSFGFCSJMF
Radio Frequency: This unit generates, uses, and can radiate radio frequency energy and, if not installed and used in accordance with the instructions,
may cause harmful interference to other devices in the vicinity. Harmful interference to other devices can be determined by turning this unit oand
on. Try to correct the interference using one or more of the following: reorient or relocate the receiving device, increase the separation between the
equipment, connect the unit to an outlet on a dierence circuit from UIBUXIJDIUIFPUIFSEFWJDFTBSFDPOOFDUFEBOEDPOTVMU.BOB.FEGPSIFMQIf
the unit isVTFEDMPTFUPSBEJPGSFRVFODZTPVSDFTFH electromagnetic security systems, cellular telephones, RFID or other in-band transmitters), it
may cause abnormal Bluetooth connection.
Be aware of the following:
tCPOTVMUXJUIZPVSQIZTJDJBOCFGPSFVTJOHUIJTEFWJDF
tTIJTEFWJDFJTOPUFòFDUJWFGPSQBJOBTTPDJBUFEXJUI$FOUSBM1BJO4ZOESPNFTTVDIBTIFBEBDIFT
tTIJTEFWJDFJTOPUBTVCTUJUVUFGPSQBJONFEJDBUJPOTBOEPUIFSQBJONBOBHFNFOUUIFSBQJFT
tTIJTEFWJDFJTBTZNQUPNBUJDUSFBUNFOUBOEBTTVDITVQQSFTTFTUIFTFOTBUJPOPGQBJOUIBUXPVMEPUIFSXJTFTFSWFBTBQSPUFDUJWFNFDIBOJTN
tSUPQVTJOHUIFEFWJDFBOEDPOTVMUXJUIZPVSQIZTJDJBOJGUIFEFWJDFEPFTOPUQSPWJEFQBJOSFMJFG
tUTFUIJTEFWJDFPOMZXJUIUIFBDDFTTPSJFTSFDPNNFOEFEGPSVTFCZUIFNBOVGBDUVSFS
The accessories may be packaged together with the device or packaged separately as the replacement.
4UPSFUIFEFWJDFBXBZGSPNIJHIUFNQFSBUVSFBOEEJSFDUTVOMJHIU4UPSBHFPVUTJEFPGTUBUFETUPSBHFUFNQFSBUVSFNBZSFTVMUJONFBTVSFNFOUFSSPSPS
device malfunction.
%POPUTIBSFUIFVTFPGUIFEFWJDFBOEJUTBDDFTTPSJFTXJUIPUIFSTUIFZBSFJOUFOEFEGPSTJOHMFQFSTPOVTF
This device contains batteries. If overheating of the device occurred, stop the operation immediately and contact customer support.
Dispose of this battery-containing device according to the local, state, or federal laws.
4LJOCVSOTNBZPDDVSBOEDIFDLUIFTLJOPGUIFUSFBUNFOUBSFBQFSJPEJDBMMZ
Adverse Reactions
:PVTIPVMETUPQVTJOHUIFQSPEVDUBOETIPVMEDPOTVMUXJUIZPVSQIZTJDJBOJGZPVFYQFSJFODFBEWFSTFSFBDUJPOTGSPNUIFEFWJDF

QUICK START GUIDE
CONTENTS: ManaSport+ Device, Applicator, Gel, Adapter, Cap, Strap, Manual
See accompanying documents / User’s Manual. CAUTION: Federal Law restricts this device
to sale by or on the order of a physician.
Date Adjusment
(Decrease)
Date Adjusment
(Increase)
Connect Applicator
Start/stop
ultrasound
Charging Port
Power on/o
Lock/Unlock
Display Symbols and Descriptions
Symbol Name Description
Low Battery
The battery runs low and needs
to be charged prior to use.
Battery Status
Shows how much charge is left in
Five levels of battery status
the battery.
Calendar Broken Star
A 20-minute treatment was not
completed on this calendar day.
Calendar Starmark
A 20-minute treatment was
completed on this calendar day.
Calendar Double
Starmark*
Two-20 minute treatments were
completed on this calendar day.
Calendar Double
Checkmark Plus*
Three or more 20-minute
treatments were completed
on this calendar day.
Ultrasound Symbol
Lock Symbol
Locks the buttons to prevent
unintended operation.
Unlock Symbol
Unlock the buttons to operate
the device.
are having your treatment.
Flashes during use to show you
Countdown Timer
Counts down from 20 minutes to
show treatment time remaining.
Treatment Complete
Automatically displays when count-
down timer reaches zero to show
that treatment is complete.
Charge the battery before use
The ManaSport+ provides non-invasive therapy of low-intensity pulsed ultrasound for the treatment of selected medical conditions such as soft tissue
injuries, shortened tendons due to past injuries and scar tissues, relief of pain, muscle spasms and joint contractures. ManaSport+ transmits a low-intensity
ultrasound signal to the patient’s treatment site through ultrasound gel. The patient will experience little or no sensation while in ultrasound mode.
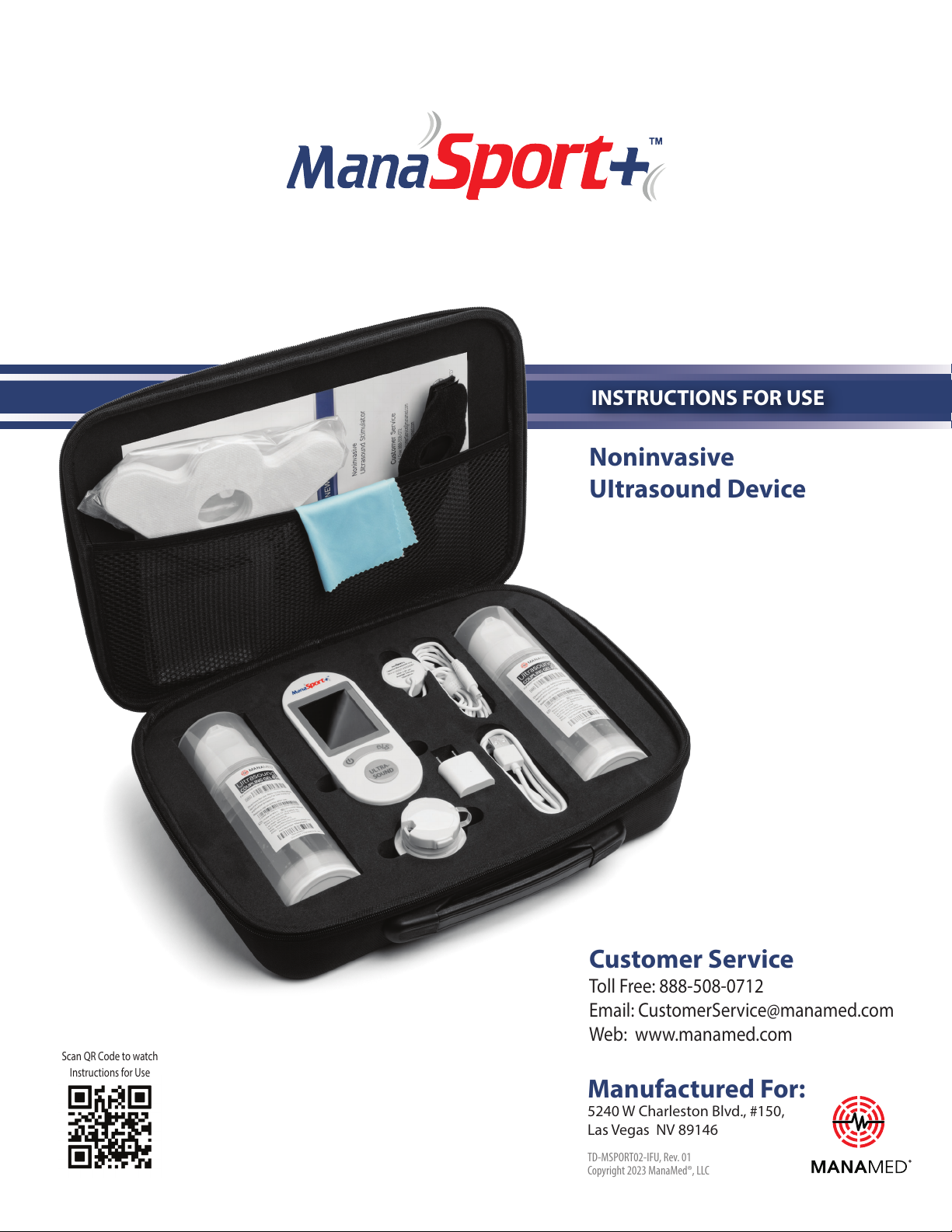
The ManaSport+ system in a carrying case consists of one ManaSport+
device and the following biocompatible components/accessories.
1) Transducer applicator connected to the ManaSport+ device to generate a low-intensity
ultrasound or stimulation signal at the treatment site.
2) Coupling gel applied to the transducer applicator to transmit the ultrasound signal
to the depth of the treatment site.
3) Strap with a cap to hold the transducer applicator down on the treatment site.
4) Battery charger/adapter to charge the subject device.
5) Sticker Patch to hold the transducer applicator down on the treatment site.
If you suspect you may be missing an item, please contact Customer Service at 888-508-0712.
When the remaining battery of the device cannot last for 20 min, its display will switch to reminding you of recharge
prior to use (Screen 1a). Plug the power supply adapter to the wall socket as well as ManaSport+ using the charging port
located at its bottom end.The BATTERY icon on the device’s display keeps ashing or steady, depending on the state of
the charge. When the battery is charging, the BATTERY icon will keep ashing (Screen 1b). Once the battery is fully
charged, the BATTERY icon will become solid (Screen 1c). Note: The device cannot be used when it is being charged.
Screen 1a Screen 1b Screen 1c
5
This manual suits for next models
1
Table of contents
Other Manamed Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual















