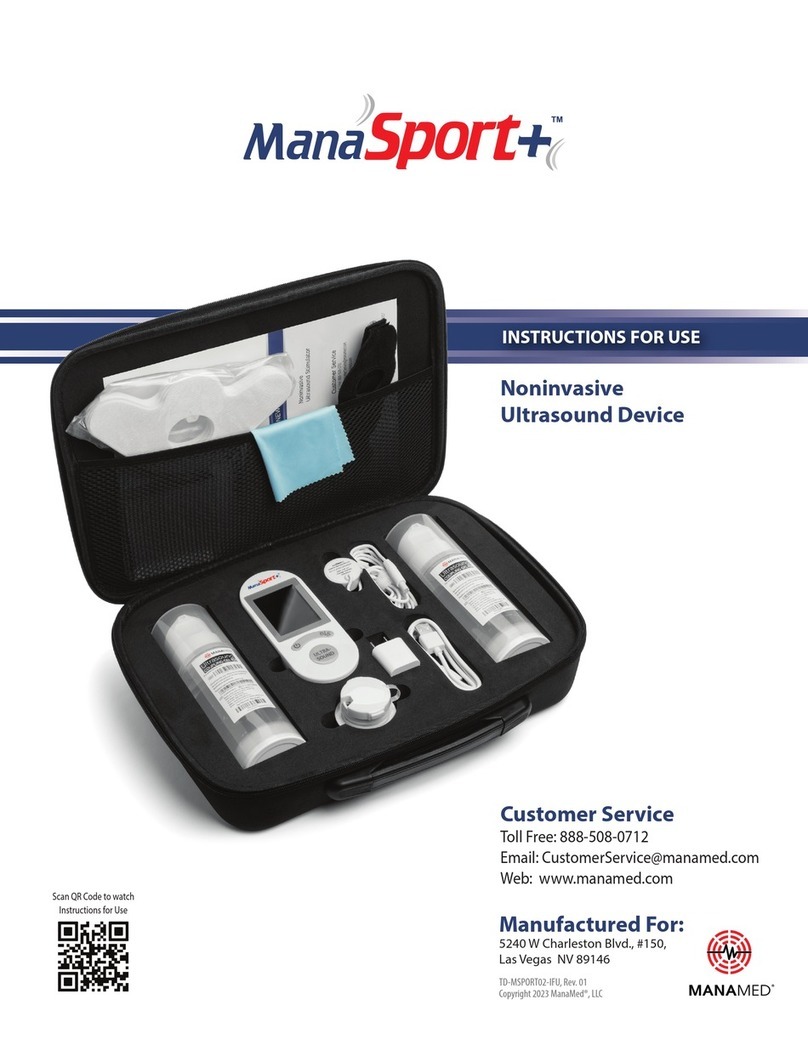
Manamed MANASPORT User manual

Noninvasive
UItrasound Stimulator
Customer Service
Toll Free: 888-508-0712
Web: www.manamed.net
Document # IFU-MSPORT01-001, Rev. 1
Copyright 2022 ManaMed™, Inc.
Manufactured For:
5240 W Charleston Blvd., Las Vegas NV 89146
INSTRUCTIONS FOR YOUR NEW MANASPORT

Table of Contents
Purpose of Device . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 3
Indications for Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 3
Contraindications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 3
Warnings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Pages 3-4
Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 4
Be Aware of the Following . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 4
Adverse Reactions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 4
Quick Start Guide . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Pages 5-7
User Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 8
Storage . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 8
Disposal . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 8
Using the AC Adapter / Battery Charger . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 8
Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 8
Ultrasound Gel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 9
ManaSport Expected Service/Shelf Life . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 9
Limited Warranty . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 9
Technical . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 9
Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 10
Instructions for reporting adverse events . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 10
Electromagnetic Compatibility Tables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Pages 11-12

PURPOSE OF DEVICE
The ManaSport is a portable and rechargeable prescriptive device.
It is intended to be used for adults only, under the direction of a medical professional. The ManaSport is not intended for Home Use.
INDICATIONS FOR USE
CONTRAINDICATIONS
Do not use this device on persons whose pain syndromes are undiagnosed.
Contraindications for the use of ultrasound include:
ǃ0WFSBOBSFBPGUIFCPEZXIFSFBNBMJHOBODZJTLOPXOUPCFQSFTFOU
ǃ0WFSUIFFZFT
ǃ0WFSPSOFBSHSPXUIDFOUFSTVOUJMCPOFHSPXUIJTDPNQMFUF
ǃ0WFSUIFSFQSPEVDUJWFPSHBOT
ǃ0WFSUIFQSFHOBOUVUFSVT
ǃ0WFSBIFBMJOHCPOFGSBDUVSF
ǃ0WFSBOBDUJWFJNQMBOUFENFEJDBMEFWJDFTVDIBTBOJNQMBOUFEEFFQCSBJOTUJNVMBUJPOEFWJDF
ǃ0OUIFCSBJOTQJOBMDPSEPSMBSHFTVCDVUBOFPVTQFSJQIFSBMOFSWFT
ǃ*TDIFNJDUJTTVFTJOJOEJWJEVBMTXJUIWBTDVMBSEJTFBTFXIFSFUIFCMPPETVQQMZXPVMECFVOBCMFUPGPMMPXUIFJODSFBTFJONFUBCPMJDEFNBOEBOEUJTTVFOFDSPTJTNJHIUSFTVMU
ǃUltrasound therapy should not be used for symptomatic local pain relief unless etiology is established or unless a pain syndrome has been diagnosed
ǃApplication over parenchymatous organs – liver, spleen, lungs, endocrine glands, gonads
ǃ#POFQSPUVCFSBODFTKVTUVOEFSUIFTLJOWFSUFCSBMTQJOPVTQSPDFTTFTBOLMFTFQJDPOEZMFT
ǃ1FSJQIFSBMOFSWFTDMPTFVOEFSUIFTLJOTVSGBDF
ǃAllergies to the applied ultrasound gels
ǃ/FBSCSBJODFSWJDBMHBOHMJBTQJOFMBNJOFDUPNZTJUFTDBODBVTFTQJOBMDPSEIFBUJOH
ǃ5PUBMIJQBSUISPQMBTUJFTXJUINFUIZMNFUIBDSZMBUFPSIJHIEFOTJUZQPMZFUIZMFOF5IFTFIBWFBIJHIDPFGGJDJFOUPGBCTPSQUJPONPSFUIBOTPGUUJTTVFBOEUIFQSPTUIFTJTDPVMEMPPTFO
due to unstable cavitation in the cement
ǃ Arthroplasties—the effect on bony ingrowth arthroplasties is not well defined; for this reason the most prudent course is avoiding ultrasonic therapy over these areas
ǃIn an area of the body where infectious disease is present
ǃBlood vessels in poor condition should not be treated as the vessel walls could rupture as a result of the treatment
ǃPatients suffering from cardiac disease should not receive treatment over the cervical ganglia, the stellate ganglion, the thorax in the region of the heart, or the vagus nerve, as a reflex
coronary vasospasm might result. Only low intensities and short treatment times should be used if these patients are treated in other areas because the stimulation of practically any
BGGFSFOUBVUPOPNJDOFSWFFTQFDJBMMZUIFWBHVTOFSWFJOUIFCPEZDPVMEDBVTFBDIBOHFJODBSEJBDSBUF
ǃPatients with thrombophlebitis or other potentially thromboembolic diseases should not be treated because a partially disintegrated clot could result in an obstruction of the arterial
supply to the brain, heart or lungs
ǃOver areas of recent bleeding or hemorrhage
ǃOver areas of active tuberculosis
WARNINGS
ǃ*GUIFUSFBUNFOUJTSFQPSUFEBTQBJOGVMPSUPPIPUBUBOZQPJOUEVSJOHUSFBUNFOUUVSOPGGEFWJDFBOESFNPWFUIFEFWJDFGSPNUIFTLJO
ǃ*OTUSVDUUIFQBUJFOUUPJOGPSNUIFQSBDUJUJPOFSJGUIFQBUJFOUGFFMTBOZQBJOPSCVSOJOHEVSJOHUSFBUNFOU
ǃ*OTUSVDUUIFQBUJFOUIPXUPUVSOPGGUIF%FWJDFBOESFNPWFUIF"QQMJDBUPSJGUIFQBUJFOUGFFMTBOZQBJOPSCVSOJOHEVSJOHUSFBUNFOU
ǃ5IF%FWJDFTIPVMECFLFQUPVUPGUIFSFBDIPGDIJMESFO
ǃ5IFEFWJDFBOEBDDFTTPSJFTBSFOPUTUFSJMF%0/05BQQMZUIJTEFWJDFBOEBDDFTTPSJFTPWFSBOPQFOXPVOEPSJOGMBNFETLJO
ǃ%0/05BQQMZEJSFDUMZPWFSBCPOFUIBUJTOFBSUIFTLJOTVSGBDF
(continued next page)
Apply stationary use of ultrasound to:
Generate deep heat within body tissues for the treatment of selected medical conditions such as the relief of pain, the relief of muscle spasms,
the treatment of joint contractures, and the local increase in circulation.
Apply continuous movement of ultrasound for:
1. Pain.
2. Pain relief, muscle spasms, and joint contractures.
3. Relief of pain, muscle spasms, and joint contractures that may be associated with:
ǃ"EIFTJWFDBQTVMJUJT
ǃ#VSTJUJTXJUITMJHIUDBMDJGJDBUJPO
ǃ.ZPTJUJT
ǃ4PGUUJTTVFJOKVSJFTBOE
ǃ4IPSUFOFEUFOEPOTEVFUPQBTUJOKVSJFTBOETDBSUJTTVFT
4. Relief of pain, muscle spasms, and joint contractures resulting from:
ǃ$BQTVMBSUJHIUOFTTBOE
ǃ$BQTVMBSTDBSSJOH
5. Localized increase in blood flow.
6. Increased range of motion of contracted joint using heat and stretch techniques.
ǃDo not use over sensitive skin areas or in the presence of poor circulation. The unattended use of this device by children or incapacitated persons may be dangerous. To reduce the
risk of buns, electric shock, and fire, this device must be used in accordance with the instructions.
ǃDo not crush the device and its accessories.
ǃCarefully examine the device and its accessories, and do not use if they show any sign of deterioration.
ǃDo not tamper with this device and its accessories in any way. There are no user serviceable parts. If for any reason they do not function satisfactorily, return to the
authorized service center at address given.
ǃ%POPUVTFBUUIFTBNFUJNFBTPUIFSUPQJDBMBOBMHFTJDT
ǃHandle ultrasound applicator with care. Inappropriate handling of the ultrasound applicator may adversely affect its characteristics.
ǃAn appropriate coupling medium should be used in order to ensure energy transmission to the tissue.
WARNINGS (continued)
Precautions
Precaution should be taken when using the device:
ǃ0WFSBOBSFBPGUIFTQJOBMDPSEGPMMPXJOHBMBNJOFDUPNZJFXIFONBKPSDPWFSJOHUJTTVFTIBWFCFFOSFNPWFE
ǃ0OQBUJFOUTXJUIIFNPSSIBHJDEJBUIFTFT
ǃ0WFSBSFBTXIFSFNFUBMQSPTUIFTJTPSPUIFSNFUBMMJDJNQMBOUTBSFFNCFEEFEJOUJTTVFXIJDINBZGPSNB
reflective surface to the ultrasound energy causing unintended irradiation of tissue and excessive heating.
ǃ0WFSBOBDVUFJOGFDUJPOPSTFQTJT
ǃ0OQBUJFOUTXJUIQFSJQIFSBMBSUFSZEJTFBTF
ǃ0WFSBEFFQWFJOUISPNCPTJT
ǃ0WFSBOBOFTUIFUJ[FEBSFBPSJODPOKVODUJPOXJUIBDPOEJUJPOUIBUDBVTFTJNQBJSNFOUPGTFOTBUJPOTVDIBTDBVTFECZDIFNPUIFSBQZ.
ǃ$BVUJPOTIPVMECFVTFEGPSQFSTPOTXJUITVTQFDUFEPSEJBHOPTFEIFBSUQSPCMFNT
ǃ$BVUJPOTIPVMECFVTFEGPSQFSTPOTXJUITVTQFDUFEPSEJBHOPTFEFQJMFQTZ
ǃ$BVUJPOTIPVMECFVTFEJGZPVIBWFBOZPGUIFGPMMPXJOH
- iGZPVIBWFBUFOEFODZUPCMFFEJOUFSOBMMZGPMMPXJOHBOJOKVSZ
- JGZPVSFDFOUMZIBETVSHFSZPSIBWFFWFSIBETVSHFSZPOZPVSCBDL
- if areas of skin lack normal sensations, such as skin that is numb.
ǃ$POTVMUXJUIZPVSQIZTJDJBOCFGPSFVTFPWFSUIFNFOTUSVBMVUFSVT
ǃ%POPUVTFUIJTEFWJDFXIJMFESJWJOHPQFSBUJOHNBDIJOFSZPSEVSJOHBOZBDUJWJUZJOXIJDIJOWPMVOUBSZNVTDMFDPOUSBDUJPOTNBZQVUUIFVTFSBUVOEVFSJTLPGJOKVSZ
ǃ,FFQUIJTEFWJDFBOEJUTBDDFTTPSJFTPVUPGSFBDIPGDIJMESFOBOEQFUT*GTXBMMPXFEHFUNFEJDBMIFMQPSDPOUBDUB1PJTPO$POUSPM$FOUFSSJHIUBXBZ
ǃ%POPUVTFUIJTEFWJDFJOIJHIIVNJEJUZBSFBTTVDIBTBCBUISPPN
ǃ4UPQVTJOHUIJTEFWJDFBUPODFJGZPVGFFMEJTDPNGPSUEJ[[JOFTTPSOBVTFBBOEDPOTVMUZPVSQIZTJDJBO
ǃ%POPUBUUFNQUUPNPWFUIFVMUSBTPVOEIFBEXIJMFUIFEFWJDFJTPQFSBUJOH
ǃFor ultrasound treatment, only use the ultrasound applicator sold with this device.
ǃWhen applying ultrasound by means of any applicator, ultrasound gel shall be used for correct passage of the ultrasound waves. It is recommended to use an FDA cleared water-based conductive
gel, specifically 40/0.&%5&$)/0-0(:*/$ Gel #, The applicators have not been tested for use with other gels or oils and can be damaged.
ǃ0OQBUJFOUTXJUIIFNPSSIBHJDEJBUIFTFT
ǃ0WFSBSFBTXIFSFUIFSFJTTFOTPSZJNQBJSNFOUPSTFOTPSZMPTT
ǃ0WFSBDVUFTLJODPOEJUJPOTTVDIBTFD[FNBEFSNBUJUJTFUD
ǃ0WFSUIFBOUFSJPSBTQFDUPGUIFOFDL
ǃ0OQBUJFOUTXIPBSFGFCSJMF
Be aware of the following:
ǃDPOTVMUXJUIZPVSQIZTJDJBOCFGPSFVTJOHUIJTEFWJDF
ǃUIJTEFWJDFJTOPUFGGFDUJWFGPSQBJOBTTPDJBUFEXJUI$FOUSBM1BJO4ZOESPNFTTVDIBTIFBEBDIFT
ǃUIJTEFWJDFJTOPUBTVCTUJUVUFGPSQBJONFEJDBUJPOTBOEPUIFSQBJONBOBHFNFOUUIFSBQJFT
ǃUIJTEFWJDFJTBTZNQUPNBUJDUSFBUNFOUBOEBTTVDITVQQSFTTFTUIFTFOTBUJPOPGQBJOUIBUXPVMEPUIFSXJTFTFSWFBTBQSPUFDUJWFNFDIBOJTN
ǃTUPQVTJOHUIFEFWJDFBOEDPOTVMUXJUIZPVSQIZTJDJBOJGUIFEFWJDFEPFTOPUQSPWJEFQBJOSFMJFG
ǃVTFUIJTEFWJDFPOMZXJUIUIFBDDFTTPSJFTSFDPNNFOEFEGPSVTFCZUIFNBOVGBDUVSFS
The accessories may be packaged together with the device or packaged separately as the replacement.
Store the device away from high-temperature and direct-sunlight. Storage outside of stated storage temperature may result in measurement error or device malfunction.
%POPUTIBSFUIFVTFPGUIFEFWJDFBOEJUTBDDFTTPSJFTXJUIPUIFSTUIFZBSFJOUFOEFEGPSTJOHMFQFSTPOVTF
This device contains batteries. If overheating of the device occurred, stop the operation immediately and contact customer support.
Dispose of this battery-containing device according to the local, state, or federal laws.
Skin burns may occur, and check the skin of the treatment area periodically.
Adverse Reactions
:PVTIPVMETUPQVTJOHUIFQSPEVDUBOETIPVMEDPOTVMUXJUIZPVSQIZTJDJBOJGZPVFYQFSJFODFBEWFSTFSFBDUJPOTGSPNUIFEFWJDF

QUICK START GUIDE
CONTENTS:
ManaSport Device, Applicator, Gel, Adapter, Cap, Strap, Manual
See accompanying documents / User’s Manual. CAUTION: Federal Law restricts this device
to sale by or on the order of a physician.
On
ULTRASOUND
19:58
Time Remaining
Decrease (-)
Increase (+)
Connect Applicator
Start/stop
ultrasound
Charging Port
Power on/off
Lock/Unlock
Display Symbols and Descriptions
Symbol Name Description
Low Battery The battery runs low and needs
to be charged prior to use.
Battery Status Shows how much charge is left in
Five levels of battery status
the battery.
Calendar Broken Star A 20-minute treatment was not
completed on this calendar day.
Calendar Starmark A 20-minute treatment was
completed on this calendar day.
Calendar Double
Starmark*
Two-20 minute treatments were
completed on this calendar day.
Calendar Double
Checkmark Plus*
Three or more 20-minute
treatments were completed
on this calendar day.
Ultrasound Symbol
Lock Symbol Locks the buttons to prevent
unintended operation.
Unlock Symbol Unlock the buttons to operate
the device.
are having your treatment.
Flashes during use to show you
Countdown Timer Counts down from 20 minutes to
show treatment time remaining.
Treatment Complete
Automatically
displays
when
count-
down timer reaches zero to show
that treatment is complete.
Charge the battery before use
Document # SPORTIFU Rev. 1
The ManaSport provides non-invasive therapy of low-intensity pulsed ultrasound for the treatment of selected sub-chronic and chronic medical conditions such as
soft tissue injuries, shortened tendons due to past injuries and scar tissues, relief of pain, muscle spasms and joint contractures. ManaSport transmits a
low-intensity ultrasound signal to the patient’s treatment site through ultrasound gel. The patient will experience little or no sensation while in ultrasound mode.
The ManaSport system in a carrying case consists of one ManaSport
device and the following biocompatible components/accessories.
1) Transducer applicator connected to the ManaSport device to generate a low-intensity
ultrasound or stimulation signal at the treatment site.
2) Coupling gel applied to the transducer applicator to transmit the ultrasound signal
to the depth of the treatment site.
3) Strap with a cap to hold the transducer applicator down on the treatment site.
4) Battery charger/adapter to charge the subject device.
If you suspect you may be missing an item, please contact Customer Service at 888-508-0712.
When the remaining battery of the device cannot last for 20 min, its display will switch to reminding you of
recharge prior to use (Screen 1a). Plug the power supply adapter to the wall socket as well as ManaSport using
the charging port located at its bottom end. The BATTERY icon on the device’s display keeps flashing or steady,
depending on the state of the charge. When the battery is charging, the BATTERY icon will keep flashing
(Screen 1b). Once the battery is fully charged, the BATTERY icon will become solid (Screen 1c). Note: The device
cannot be used when it is being charged.
Screen 1a Screen 1b Screen 1c

QUICK START GUIDE (continued)
Operation 4b
Operation 4a
Operation 4c
Preparation before use:
ǃRemove the cap from the gel bottle, and hold the transducer applicator, so the cable is down and the smooth side of the applicator is up. Press down the gel bottle nozzle to pump gel onto the smooth side of the
applicator, as shown in the following Operation 4a. You only need on full pump of gel on the applicator (Note: For the first time, you may need to press the gel bottle nozzle a couple of times to start the gel flowing);
ǃPlace the transducer applicator with the gel side down into the port of the cap, and the gel will touch the skin over your treatment site, as shown in the following Operation 4b;
ǃAlign the lead wire coming out of the transducer applicator with the notch of the cap cover, and close the cap cover, as shown in the following Operation 4c
(Note: The other end of the lead wire of the transducer applicator is connected to the top port of the device);
IMPORTANT:
Patients should ensure the
entire applicator surface is
covered with the ultrasound gel.
Patient should also ensure
complete skin contact with the
treatment applicator before
beginning therapy.
Operation 2a
Operation 2e
Operation 2b
Operation 2d
How to install the strap with a cap:
The strap is suitable for a treatment site of extremities. Position the strap with the cap facing up, as shown in the following Operation 2a;
ǃPull the long end of the strap through the plastic loop, as shown in the following Operation 2b;
ǃUse 2 fingers of yours to squeeze the cap tabs together to open the cap cover, as shown in the following Operation 2c;
ǃSlide the strap and place the port of the cap over the treatment site marked with an ‘X’ by your physician/doctor, as shown in the following Operation 2d
(Contact your physician/doctor if you are not sure where to locate your treatment site);
ǃTighten the strap by pulling on the long end and fasten the strap in place, as shown in the following Operation 2e (Note: Do not make the strap too tight);
Operation 2c
The strap can provide you with a hand-free treatment. However, if you prefer to continuously move the transducer applicator throughout
your treatment area, you can skip operations 2a to 4c, and only refer to Operation 4a at the bottom of this page.
Specifically, please refer to the detailed instructions below for continuously moving the applicator..
a. Apply the ultrasound gel. The entire treatment area should be covered with the ultrasound gel, and the transducer applicator should
also always be covered with the ultrasound gel.
b. Position the transducer applicator flat against the skin and keep transducer applicator constantly moving, slowly and with constant
pressure, during treatment.
c. The treatment area should not exceed 2 to 3 times the size of the transducer applicator.

QUICK START GUIDE (continued)
Screen 5a Screen 5b Screen 5c
Screen 5d Screen 5e Screen 5f
To avoid the unintended operation, the device will automatically be locked after 60 seconds of no operation, or you
could click the Lock/Unlock button to lock the device (Screen 5g). After the 20-minute timer counts down to zero, you
can see a “Treatment Completed” screen (Screen 5h). It means a 20-minute treatment of Ultrasound is complete. After
showing the “Treatment Completed” screen for 20 seconds, the device will turn off automatically (alternatively, you
could press and hold the ON/OFF button to turn off the device). Please be aware that you still can press and hold the
ON/OFF button to turn off the device, even though the device is locked.
ManaSport Quantity of Treatment and Frequency of Use
ManaSport should be used for 20 minutes per day or as prescribed by your doctor. The ultrasound will be
automatically stopped after the maximum timer of 20 minutes runs out. It is very important to follow your
physician/doctor’s protocol to get the full medical benefit of the treatment. If you have concerns about your
treatment, please contact your doctor directly. The device is a single patient reusable device. This device is
prescription only and may not be used without a physician’s order.
Note: To ensure the patient safety, we implement the maximum temperature protection of 43º C. When the
transducer applicator senses the surface temperature reaching 43º C., the output of the ultrasound will stop and
automatically resume when it cools down.
Screen 5g Screen 5h
Click ULTRASOUND to navigate
Click ON/OFF to conrm
Screen 7a
Data Record
Your daily use time of Ultrasound is recorded, as shown on Screen 7a. When a 20-minute treatment is completed, a red starmark will
show on the calendar date. When two 20-minute treatments are completed, two red starmarks will show on the calendar date. When
three or more 20-minute treatments are completed, two red starmarks and one plus will show on the calendar date. When a
20-minute treatment is not completed, a grey broken starmark will show on the calendar date.
Please refer to the above section of “Use of Ultrasound function” for how to view the data recorded. After the “Set
Successfully” screen of Date and Time, the device will automatically proceed to the next screen of Ultrasound
calendar/summary (Screen 7a) and last for 5 seconds. Clicking the + or – button on the right side of the device can
show different-month screens of Ultrasound calendar/Summary. It is worth noting that no patient information (such
as age and history) is recorded and stored, so there is no issue of privacy or security.
Use of Ultrasound function:
Your physician/doctor may have marked your treatment site with an ‘X’.
This is the spot to place the transducer applicator to treat your
symptom by using ultrasound. Contact your physician/doctor if you are
not sure where to treat your symptom.
After the above steps are done, you can click the ON/OFF button on the
device to turn it on: After showing the following display screen (Screen
5a) for 2 seconds, the device will then go to the next screen (Screen 5b)
of Date and Time automatically. Follow the instruction of the screen to
conform or change the time and date, and you will see the “Set
Successfully” screen of Date and Time (Screen 5c). Note: The display
screen of Date and Time will only appear for the first time of power on,
or by pressing the ON/OFF and ULTRASOUND buttons simultaneously.
After the “Set Successfully” screen of Date and Time, the device will
automatically proceed to the next screen of Ultrasound
calendar/summary (Screen 5d) and last for 5 seconds. Clicking the + or –
button on the right side of the device can show different-month screens
of Ultrasound calendar/Summary. Note: The screen of Ultrasound
calendar/summary will appear each time when the device is turned on, or
by clicking ON/OFF after the device is on.
After the screen of calendar/summary (Screen 5d), the device will
automatically proceed to the next screen of Ultrasound standby (Screen
5e). Follow the instruction on this screen to add more ultrasound gel on
the transducer applicator if needed, and click the ULTRASOUND button
to start the Ultrasound function (Screen 5f). Relax and enjoy the 20
minute treatment of ultrasound provided.

USING THE AC ADAPTER /
BATTERY CHARGER
IMPORTANT:
Charge device before first use.
WARNING:
Use only the charger provided by ManaMed
™
. The use of the wrong charger
can cause excessive heat, damage to the circuit and shorten the life of the battery.
CHARGING:
Plug in the power supply adapter to the wall socket using the plug located at the
bottom end of the device. The BATTERY icon on the device will keep flashing or steady, depending on
the state of the charge. When the battery is charging, the BATTERY icon will keep flashing. Once the
battery is fully charged, the BATTERY icon will become solid.
Note: The device cannot be used when it is being charged.
CLEAN AFTER TREATMENT
ǃ6TFZPVSUXPGJOHFSTUPTRVFF[FUIFDBQUBCTUPHFUIFSUPPQFOUIFDBQDPWFSBOEUBLFPVU
the ultrasound head; Wipe and clean the ultrasound gel on the ultrasound head with a blue
cloth or paper towel;
ǃ8JQFBOEDMFBOUIFVMUSBTPVOEHFMJOUIFDBQXJUIBCMVFDMPUIPSQBQFSUPXFM
CLEANING AND MAINTENANCE
UNIT DEVICE:
To clean the device, please wipe it with a soft cloth. You can use wipe of
water, alcohol, and mild detergent for cleaning first, and then use the dry cloth to wipe it again.
TRANDUCER APPLICATOR:
After the treatment is completed, wipe the
transducer applicator with a soft cloth or paper towel to remove the ultrasound gel. You can
use wipe of water, alcohol, and mild detergent for cleaning first, and then use the dry cloth
to wipe it again.
Unit must be completely dry prior to use. To ensure that, leave the device in the OFF
position and disconnected from the wall outlet for at least 30 minutes (and as long as
necessary for the unit to dry completely) after cleaning or disinfecting.
ǃ%POPUVTFIBJSESZFSUPBDDFMFSBUFESZJOH
ǃ%POPUQMBDFUIFEFWJDFPOUPQPGPSJOGSPOU
of portable or stationary radiators to
accelerate drying.
ǃ%POPUVTFBCSBTJWFDMFBOFST
USER MAINTENANCE
Contains no serviceable parts.
Contact ManaMed Customer Service at 888-508-0712.
Inspect the unit and all components for any damage that may have occurred during
shipping or general handling prior to each use (for example, frayed or cut charging cord,
cracked plastic housings,, etc).
Refer to image of ManaSport for description of all components.
Do not attempt to connect the wall supply if any damage is noticed.
Avoid subjecting the unit to shocks, such as dropping the device.
Battery is not replaceable; replacement units are available through customer service.
Contact ManaMed to receive replacements instructions for any damaged items.
STORAGE
Store in a dry location between +10°C (50°F) and +40°C (104°F).
Do not expose to heat exceeding 50°C (122°F) for extended periods of time.
Do not store items in direct sunlight.
DISPOSAL
This unit is an electromechanical device that includes printed circuit boards and
rechargeable CBUUFSJFT%POPUEJTDBSEJOMBOEGJMM$POTVMUMPDBMDPVOUZSFRVJSFNFOUT
for proper disposal instructions.
This unit contains rechargeable batteries. Do not discard the unit in regular waste.
Bring the unit to your local recycle center or contact ManaMed.
The use of accessories, power supplies and cables other than those
specified, with the exception of components sold by the manufacturer
of the ManaSport as replacement parts, may result in increased
emissions or decreased immunity of the ManaSport .
%FTJHOBUFT$MBTT**NFEJDBMFMFDUSJDBMFRVJQNFOU
This unit is an electromechanical device that includes printed circuit
boards and rechargeable batteries. Do not discard in landfill. Consult
MPDBMDPVOUZSFRVJSFNFOUTGPSQSPQFSEJTQPTBMJOTUSVDUJPOT
This symbol designates the degree of protection against electrical
shock from the wrap as being a type B applied part.
Consult instructions for use.
CAUTION: Federal Law restricts this device to sale by or on the order
of a physician.
WARNING: This device is not protected against water.
&RVJQNFOU JT OPU TVJUBCMF GPS VTF JO UIF QSFTFODF PG
flammable anesthetic mixture with air, oxygen, or nitrous
oxide. The rechargeable batteries supplied in this unit
are not field replaceable. If you have any issues please
contact 888-508-0712.for a replacement unit.

Limited Warranty
ManaMed (“Seller”) warrants that the original purchaser (“Purchaser”) of its ManaSport purchased by the Purchaser directly from Seller (“Device”) that the Device confirms to
Seller’s manufacturing specifications. The warranty will be one year for the date of purchase. In the event of a breach of this warranty, with a 30-day written notice, Seller
will, at its sole option, either repair or replace the Device or issue a refund at the original purchase price. This warranty is null and void if the Device is resold or a transfer of
the Device by Purchaser to any other person or entity. Seller expressly disclaims any and all other warranties, either expressed or implied, relating to the system or its
performance, including, without limitation, any implied warranty of merchantability and any implied warranty of fitness for a particular purpose. This limited warranty does not
cover damages due to eternal causes, including, without limitation, accident, usage not in accordance with product instructions, misuse, neglect, alteration, or repair.
ManaSport Expected Shelf Life
The shelf life of ManaSport is not applicable because of low likelihood of time-dependent product degradation. Among all the accessories marketed with ManaSport, the
ultrasound gel may have a certain shelf life, and you can find the corresponding shelf life or expiration date on the bottle of ultrasound gel.
ManaSport Expected Service Life
The expected service life of ManaSport and its accessories is 365 days from the initial treatment. Once ManaSport reaches 365 days, it will provide no further treatment.
ULTRASOUND GEL
Ultrasound gel is provided for use with ManaSport. Patients are instructed to place gel on the transducer applicator every time you use ManaSport. This gel allows the
ultrasound signal to reach the depths of the treatment site through your skin. If the transducer applicator is not properly applied, the patient will receive an alert from the
device. For the best result, only use the gel supplied. If you need more gel, please contact SONOMED TECHNOLOGY, INC., P.O. BOX 10489, State College, PA 16805, or by
calling 908-722-4549, Reference Gel #K883917.
Caution: Some patients may experience mild skin irritation to the gel. If you feel your skin is sensitive to the gel,
please contact your physician or use a different FDA-cleared ultrasound gel.
Technical Specifications
Ultrasound frequency: 1.5 +/- 20% MHz
Ultrasound Duty cycle: 100%
Ultrasound effective radiating area (ERA): 3.9 +/- 20% square cm (cm2)
Ultrasound power: 0.60 +/- 20% watts (W)
Ultrasound spatial average temporal avg. (SATA): 0.16 +/- 20% W/cm2
Ultrasound beam non-uniformity ratio (BNR): 4.0 maximum
Ultrasound beam type: Collimated
Battery: 3.7 VDC Lithium battery
Input Voltage (USB) of battery charger: 5.0 VDC
TECHNICAL DATA
Specifications:
Dimensions: 14cm x 5.6cm x 2.4cm
Weight: Approx. 0.13 kg
SYSTEM OPERATING ENVIRONMENT:
Temperature: +10°C (50°F) to +40°C (104°F)
Humidity: 30%-75%. Keep dry.
Source of Power: DC 5 V or Inner Battery (3.7 volt Li-ion battery)
CAUTION:
Charge batteries using only the
power source provided by ManaMed.
POWER SUPPLY:
Class II, input: 100 - 240 Vac, 50 - 60 Hz, output: 5 V @ 1 Amp)
Use only UL/60601-1 approved power supplies from ManaMed for use in hospital settings.
Maximum cable length for the power cable: 1 meter
TOLERANCES:
Output +_20%.
BATTERY CHARGE:
Takes approximately 4 hours
(from depleted state)..
BATTERY RUN TIME:
10 hours

Troubleshooting
ManaSport will alert you by displaying an alert screen, if something is not working properly. See the following table for common alerts and problems as well as what to do
if you get an alert or problem. If you get any other problem, do not try to fix ManaSport by yourself and contact customer service at 888-508-0712.
Alert / Problem
Blank screen, and ManaSport
does not turn on.
The battery area on ManaSport
or the battery charger gets
excessively warm.
What does this mean?
Contact customer service:
ManaSport will display the yellow “Contact manufacturer”
screen when detecting that it is not working properly.
Low battery:
ManaSport will display the “Low Battery” screen,
when the battery level is very low. You are not
able to start treatment or view history.
The battery may be completely discharged or the
ManaSport device has malfunctioned.
The battery or charger may be malfunctioning.
What should I do?
Call customer service at 888-508-0712.
Do not try to fix ManaSport by yourself.
You must charge ManaSport. Plug ManaSport into a
power source with the provided charger.
Plug ManaSport into a power source with the provided
charger and fully charge its battery. If ManaSport still does
not respond, contact customer service at 888-508-0712.
Stop charging ManaSport and
contact customer service at 888-508-0712.
Contact Manufacturer
Recharge prior to use
Instructions for reporting adverse events:
MedWatch is the Food and Drug Administration's (FDA) program for reporting serious reactions, product quality problems, therapeutic inequivalence/failure,
and product use errors with human medical products, including drugs, biologic products, medical devices, dietary supplements, infant formula, and cosmetics.
If you think you or someone in your family has experienced a serious reaction to a medical product, you are encouraged to take the reporting form to your
doctor. Your health care provider can provide clinical information based on your medical record that can help FDA evaluate your report.
However, we understand that for a variety of reasons, you may not wish to have the form filled out by your health care provider, or your health care provider
may choose not to complete the form. Your health care provider is not required to report to the FDA. In these situations, you may complete the Online
Reporting Form yourself.
You will receive an acknowledgement from FDA when your report is received. Reports are reviewed by FDA staff. You will be personally contacted only if we
need additional information.
Submitting Adverse Event Reports to FDA
Use one of the methods below to submit voluntary adverse event reports to the FDA:
Report Online at www.accessdata.fda.gov/scripts/medwatch/index.cfm?action=reporting.home.
Consumer Reporting Form FDA 3500B. Follow the instructions on the form to either fax or mail it in for submission. For help filling out the form, see
MedWatchLearn. The form is available at www.fda.gov/downloads/aboutFDA/reportsmanualsforms/forms/ucm349464.pdf. Call FDA at 1-800-FDA-1088
to report by telephone.
Reporting Form FDA 3500 commonly used by health professionals. The form is available at
www.fda.gov/downloads/aboutFDA/reportmanualsforms/forms/ucm163919.pdf.
ELECTROMAGNETIC COMPATIBILITY (EMC)
TABLES - RF EMISSIONS CLASS B
GUIDANCE AND MANUFACTURER’S DECLARATION - ELECTROMAGNETIC EMISSIONS
The ManaSport is intended for use in the electromagnetic environment specified below.
The customer or the user of the ManaSport should assure that it is used in such an environment.
RF Emissions CISPR11
Harmonic Emissions IEC
61000-3-2
Voltage Fluctuations IEC
61000-3-3
RF Emissions CISPR11
Class B
Class A
Complies
Group 1
ManaSport is suitable for use in all establishments, including domestic establishments and those directly connected to the
public low-voltage power supply network that supplies buildings used for domestic purposes.
ManaSport uses RF energy only for its internal function. Therefore, its RF emissions are very low and are not likely to cause
any interference in nearby electronic equipment.
Emissions Tests Compliance Electromagnetic Environment Guidance
GUIDANCE AND MANUFACTURER’S DECLARATION - ELECTROMAGNETIC IMMUNITY
The ManaSport is intended for use in the electromagnetic environment specified below.
The customer or the user of the ManaSport should assure that it is used in such an environment.
Electrostatic
Discharge (ESD)
IEC 61000-4-2
Electrical Fast
Transient/Burst
IEC61000-4-4
Surge
IEC61000-4-5
±1kV differential
mode
±2kV common mode
±1kV differential
mode
±2kV common mode
Mains power quality should be that of a typical commercial or hospital environment.
Mains power quality should be that of a typical commercial or hospital environment. If the user of the
ManaSport requires continued operation during power mains interruptions, it is recommended that the
ManaSport be powered from an uninterrupted power supply or a battery.
Voltage
dips, short
interruptions
and voltage
variations on
power supply
input lines
IEC61000-4-11
Power Frequency
(50/60Hz)
Magnetic Fields
IEC61000-4-8
30 A/m at
50 or 60 Hz
30 A/m at
50 or 60 Hz
Power frequency magnetic fields should be at levels characteristic
of a typical location in a typical commercial or hospital environment.
<5%UT(>95% dip in UT)
for 0.5 cycle
40%UT(60% dip in UT)
for 5 cycles
70%UT(30% dip in UT)
for 25 cycles
<5%UT(>95% dip in UT)
for 5 seconds
NOTE: UTis the a.c mains voltage prior to application of the test level.
<5%UT(>95% dip in UT)
for 0.5 cycle
40%UT(60% dip in UT)
for 5 cycles
70%UT(30% dip in UT)
for 25 cycles
<5%UT(>95% dip in UT)
for 5 seconds
±2kV for power
supply lines
±1kV for input/
output lines
±2kV for power
supply lines
±1kV for input/
output lines
Mains power quality should be that of a typical commercial or hospital environment.
Floors should be wood, concrete or ceramic tile. If floors are covered with synthetic material,
the relative humidity should be at least 30%.
±8kV contact
±15kV air
±8kV contact
±15kV air
Immunity
Test IEC 60601 Test
Level Compliance
Level Electromagnetic Environment Guidance
The ManaSport is a portable and rechargeable prescriptive device.
Warning: Don’t use near active HF surgical equipment and the RF shielded room of an ME system for magnetic resonance imaging, where the intensity of EM disturbances is high.
Warning: Use of this equipment adjacent to or stacked with other equipment should be avoided because it could result in improper operation. If such use is necessary, this equipment and the other equipment should be observed to verify that they
are operating normally.
Warning: Use of accessories, transducers, and cables other than those specified or provided by the manufacturer of this equipment could result in increased electromagnetic emissions or decreased electromagnetic immunity of this equipment and
result in improper operation.
Warning: Portable RF communications equipment (including peripherals such as antenna cables and external antennas) should be used no closer than 12 in (30 cm) to any part of the equipment, including cables specified by the manufacturer.
Otherwise, degradation of the performance of this equipment could result.
Technical description:
1. All necessary instructions for maintaining BASIC SAFETY and ESSENTIAL PERFORMANCE with regard to electromagnetic disturbances for the excepted service life.
2. Guidance and manufacturer’s declaration-electromagnetic emissions and Immunity.

ELECTROMAGNETIC COMPATIBILITY (EMC)
TABLES - RF EMISSIONS CLASS B (continued)
GUIDANCE AND MANUFACTURER’S DECLARATION - ELECTROMAGNETIC IMMUNITY
RECOMMENDED SEPARATION DISTANCE BETWEEN PORTABLE AND MOBILE RF COMMUNICATIONS EQUIPMENT AND
THE ManaSport
The ManaSport is intended for use in the electromagnetic environment specified below.
The customer or the user of the ManaSport should assure that it is used in such an environment.
Conducted RF
IEC61000-4-6
Portable and mobile RF communications equipment should be used no closer to any part of the ManaFuse,
including cables, than the recommended separation distance calculated from the equation applicable
to the frequency of the transmitter.
Recommended separation distance
150 KHz to 80 MHz
80 MHz to 800 MHz
800 MHz to 2.5 GHz
Radiated RF
IEC61000-4-3
NOTE 1: At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people.
The ManaSport is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the ManaSport can help
prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the ManaSport as
recommended below, according to the maximum output power of the communications equipment.
Separation distance according to frequency of transmitter
m
Rated maximum output
power of transmitter
W
0.01
0.1
1
10
100
0.12
0.38
1.2
3.8
12
0.12
0.38
1.2
3.8
12
0.23
0.73
2.3
7.3
23
150 KHz to 80 MHz 80 MHz to 800 MHz 800 MHz to 2.5 GHz
a
Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM and FM radio broadcast and
TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to the fixed RF transmitters, an electromagnetic site survey should
be considered. If the measured field strength in the location in which the ManaSport is used exceeds the applicable RF compliance level above, the ManaSport should be
observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as reorienting or relocating the ManaFuse.
b
Over the frequency range 150 kHz to 80 MHz, field strengths should be less than [V1] V/m.
NOTE 1: At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people.
For transmitters rated at a maximum output power not listed above, the recommended separation distance in meters (m) can be estimated using the equation applicable to
the frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer.
where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter
manufacturer and d is the recommended separation distance in meters (m).
Field strengths from fixed RF transmitters, as determined by an electromagnetic site survey
a
, should be less than
the compliance level in each frequency range
b
.
Interference may occur in the vicinity of equipment marked with the following symbol:
3 V/m
80 MHz to 2.5
GHz
10 V/m
3Vrms
150 kHz to 80
MHz
3Vrms
Immunity
Test IEC 60601
Test Level Compliance
Level Electromagnetic Environment Guidance
d = 1.2 √P
d = .35 √P
d = .70 √P
d = 1.2 √Pd = .35 √Pd = .70 √P
Table of contents
Other Manamed Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual