Mediblink M170 User manual

1
EN
Mediblink Pulse Oximeter M170
INSTRUCTIONS FOR USE
Please read the instructions for use
carefully before using the product
Instructions for use version: 1.1
Issue date: 17.04.2020
Date of last change: 04.02.2022

2
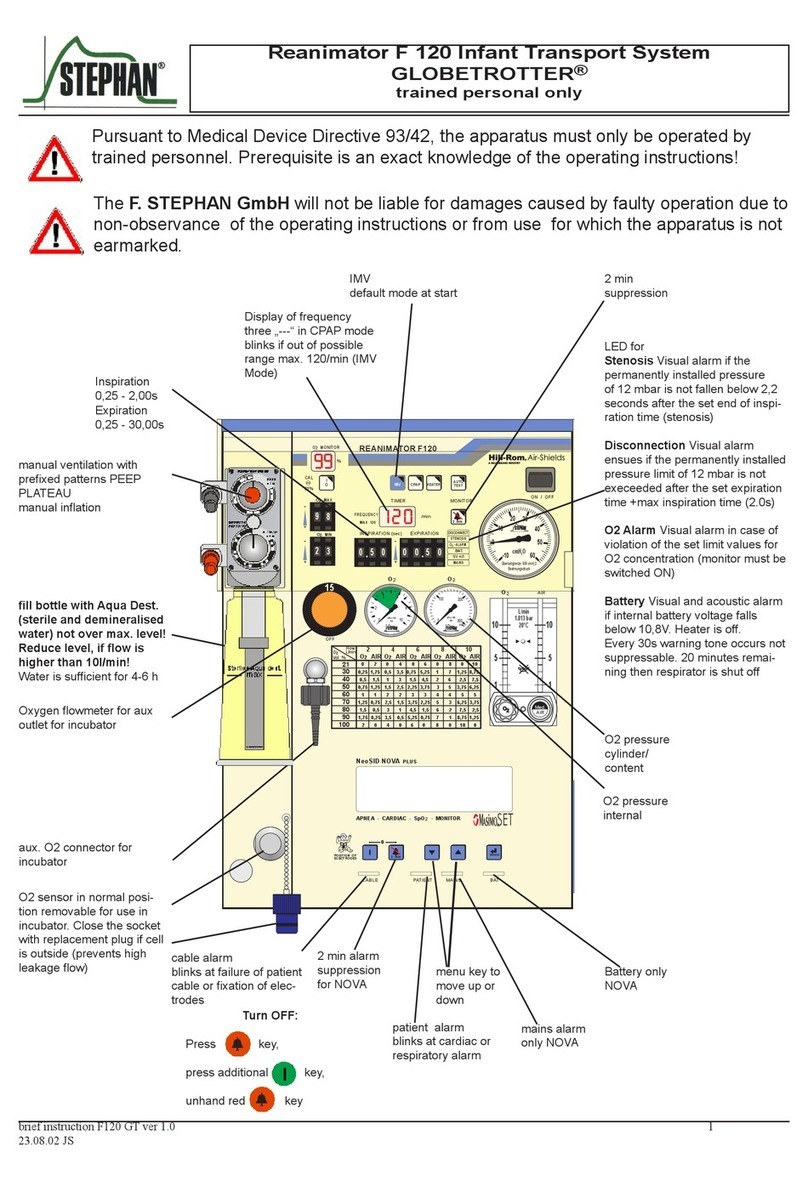
1. Product Introduction
and operation Guide
1.1 Front View
SpO2 value
Button
Empty battery indicator
Perfusion Index
Pulse bar graph
Pulse rate value
Figure 1: Front View of M170 (Model: YM103)
1.2 Operation Method
A. Open the battery cover, and put the two AAA
batteries into the battery compartment in correct
polarities, then put the cover back in place;
B. Press the bottom of the equipment and open the
probe, then insert one finger into the probe;
C. Press the button to turn the equipment on, and the
measure interface will appear;
D. After about 8 seconds, the measurement result can
be read directly from the display screen;
E. Before reading the parameters, make sure that sta-
ble numbers of the pulse oximeter interface were
sustained for more than 4 seconds;
F. The equipment will turn off automatically within 8
seconds after the finger was put off the probe.

3
1.3 Battery Installation
A. Put the two AAA batteries into battery compartment
in correct polarities (Figure 2).
B. Push the battery cover horizontally in direction of
the arrow as shown on battery cover.
WARNINGS
• Battery polarities should be correctly installed, oth-
erwise damage may be caused to the equipment.
• Please remove the batteries, if the equipment will
not be in use for a long time.
Figure 2: Battery Installation
1.4 Lanyard Installation
A Pass the thinner end of the lanyard through the
hanging hole;
B Pass the thicker end of the lanyard through the
thinner end, and tighten the lanyard (Figure 3).

4
Figure 3: Lanyard Installation
1.5 Attention for Operation
A. Before use, check and confirm that the people or
finger size are applicable;
B. Before use, check and confirm that there are
non-combustible materials in the environment,
avoid high or low temperature and humidity, and
pay attention to the following:
a) To avoid glare and direct sunlight exposure;
b)
To avoid radiation, infrared or ultraviolet radiation;
c) Avoid contact with the organic solvent, mist,
dust, corrosive gases;
C. The equipment should not be used at a location of
limb tied with arterial canal, or near blood pressure
cuff, or when receiving intravenous injection;
D. The equipment may not work normally on micro-
circulation barrier patients. Warming or rubbing
the finger, or re-positioning the equipment could
improve the measurement;
E. The ray between photo detector and light emitting
diode should cross patient’s arteriole;

5
F.
The patient should not use enamel or other makeup;
G. Avoid inserting a wet finger into the probe.
Notes:
A. The user should fully insert the finger into the probe
(Figure 4);
B. It is recommended to let the LED light shine directly
on the nail;
C. Don’t shake the finger and try to keep the patient
still during the measurement.
Figure 4: Finger Placement Diagram
1.6 Functions and Menu Operation
1.6.1 The Button Operation Rules
Long-press functions include: entering menus, ac-
tivating item’s submenu, confirming setting values,
and exiting item’s submenu; Short-press functions
are polling menu items and viewing the setting values

6
of items. It should be noted that long-press means
pressing the key for about 2 seconds, and short-press
means pressing the key for less than 0.5 second.
1.6.2 Menu Operation
Activate the menu
After the oximeter is turned on, long-press the power
button to activate the menu, then short-press the button
to view the setting values of each item. If the user wants
to change the setting value of the item, long-press to en-
ter the item’s submenu, and the parameter value starts
to flash, short-press to traverse the parameter value until
the parameter value required by the user is selected,
long press to confirm and exit the submenu.
Item 1: Setup the LED Display Brightness
The first item is to setup the display brightness. Long-
press the button to select a brightness level ranging
from 1 to 3. The greater the value, the greater bright-
ness of the display.
Item 2: Setup the SpO2 Alarm Limits
The second item is to setup the SpO2 alarm limits. For
example: When SpO2 High limit is set to 96, an alarm
will be issued when the SpO2 value is higher than

7
96, and when SpO2 Low limit is set to 94, an alarm
will be issued when the SpO2 value is lower than 94.
Item 3: Setup the PR Alarm Limits
The third item is to setup the PR alarm limits. For
example: When PR High limit is set to 130, an alarm
will be issued when the PR value is higher than 130,
and when PR low limit is set to 50, an alarm will be
issued when the PR value is lower than 50.
Item 4: Turn Alarm On/Off
The forth item is long-press to turn Alarm On/Off.
Item 5: Check the software version
The fifth item is to view the software version.

8
2. Specications
2.1Classication
Type of protection against electric shock:
(Internally powered equipment)
Degree of protection against electric shock:
Type BF - Applied part
Operating mode: Spot checking
Degree of protection against hazards of explosion:
IP22
2.2 Power Requirements
Specification of battery: Two AAA (LR03)
Operating current: 25–50 mA
2.3PhysicalSpecications
Width x Height x Depth: 57 x 30 x 31 mm
Net Weight: 28 g (product without batteries)
2.4MeasurementSpecications
SpO2 declared accuracy: 70%~100%: ±2 digits
0%~69%: unspecified
SpO2 Display Range: 30%~99%
SpO2 Resolution: 1%
PR declared accuracy: 25-250 bpm: ±3digits
PR Resolution: 1 bpm

9
2.5EnvironmentalSpecications
Temperature
Operating: +41~+104°F / +5°~+40°C
Storage/Transportation: 40~+140°F / -40°~+60°C
Humidity
Operating: 10~95%, noncondensing
Storage/Transportation: 10~95% noncondensing
Atmosphere Pressure
Operating: 70-106 kPa
Storage/Transportation: 50~107.4 kPa
2.6 Display
Display Type: 1.5” LED Display
Display Color: White
Display Content: SpO2%, Pulse Rate, Pl%,
Bar Graph, Battery Indicator
Notes:
1. The claim for oxygen saturation accuracy should
be supported by clinical studies covering the
entire claimed range. The fraction of inspired
oxygen (FiO2) delivered to test subjects is varied
to achieve a series of targeted steady-state satu-
ration periods over the specified SpO2 accuracy
range (e.g. 70% to 100%), then the SpO2 accuracy
is calculated by comparing SpO2 readings of the
pulse oximeter to the values of SpO2 determined
with a Co-Oximeter.

10
2. The clinical trial included 11 subjects, including 6
males and 5 females, with an age range of 18 to 46
years, the subjects skin color included dark black,
medium black, light color and white.
3. Maintenance, Cleaning,
Disinfection
3.1 Maintenance
The equipment’s design life expectancy is about 2
years, keep your equipment and accessories free of
dust and dirt and follow these rules:
A. Please clean the equipment before use, according
to chapter 6.2; Remove the batteries inside the bat-
tery cassette, if the equipment will not be operated
for a long time;
B. Replace the batteries in time when the battery
voltage indicate lamps were empty;
C. It is recommended that the equipment should be
kept in a dry environment with no corrosive gases
and good ventilation anytime. The moisture and
high-light environments will affect its lifetime and
even might damage the equipment.
D. It is best to preserve the product in a place where
the temperature is between -20 to 60°C and the
relative humidity is less than 95%.

11
E. The packed equipment can be transported by ordi-
nary conveyance. The equipment must not be trans-
ported mixed with toxic, harmful, corrosive materials.
WARNING
• No modification of this equipment is allowed.
3.2 Cleaning
Your equipment should be cleaned on a regular basis.
If there is heavy pollution or lots of dust and sand in
your place, the equipment should be cleaned more
frequently. Before cleaning the equipment, consult
your hospital’s regulations for cleaning the equipment.
Recommended cleaning agents are:
a) Mild soap (diluted)
b) Ethanol (70%).
To clean your equipment, follow these rules:
a) Shut down the pulse oximeter;
b) Clean the display screen using a soft, clean
cloth dampened with a glass cleaner;
c) Clean the exterior surface of the equipment
and probe using a soft cloth dampened with
the cleaner;
d) Wipe off all the cleaning solution with a dry cloth
after cleaning if necessary;
e) Dry your equipment in a ventilated, cool place.
To avoid damage to the equipment, follow these rules:

12
CAUTIONS
• Always dilute according the manufacturer’s instruc-
tions or use lowest possible concentration.
• Do not immerse part of the equipment in the liquid.
• Do not pour liquid onto the equipment or accesso-
ries.
• Never use abrasive materials (such as steel wool
or silver polish), or erosive cleaners (such as ace-
tone or acetone-based cleaners).
• If you spill liquid onto the equipment, contact us or
your service personnel.
3.3 Disinfection
Clean the pulse oximeter before disinfecting it. The
recommended disinfectant is 70% ethanol. Disinfec-
tion steps are the same as cleaning.
CAUTION
• Never use ETO or formaldehyde for disinfection.
3.4 Disposal
Dispose of the pulse oximeter in accordance with
local environment and waste disposal laws and reg-
ulations.

13
4. Accessories
•One lanyard
•Two AAA batteries
•User manual
5. Troubleshooting
Trouble Possible Reason Solutions
The device
can not be
turned on
The batteries are
drained away or
almost drained
away.
Replace batteries
The battery
installation is
incorrect
Install the battery
over again.
The device works
abnormally
Please contact the
product distributor
The SpO2
and PR
are not
displayed
normally
The finger size is too
big or small
Select the suitable
size finger to
measure
Excessive ambient
light
Avoid the
excessive ambient
light irradiation
User’s blood
perfusion is very low
Warm the finger
and try again

14
Trouble Possible Reason Solutions
The display
is off
suddenly
The device was
set to shut down
automatically in
8 seconds when
there is correct
physiological signal
Normal
The battery is almost
drained away Replace batteries
The SpO2
and PR
are not
displayed
stable
The finger is not
inserted deep
enough
Replace the finger
and try again
The finger is shaking
or the body is
moving
Try to keep still
Not used in the
work environment
required by this
manual
Please use in
normal working
environment
The device works
abnormally
Please contact the
product distributor

15
7. Warranty
Product: Mediblink Pulse Oximeter M170
Manufactured for (also EU importer):
Mediblink d.o.o., Gubčeva cesta 19, 8210 Trebnje,
Sellers name, address, signature and stamp*:
Date of extradition/sales*:
*If the invoice is accompanied by this warranty, and if all above informa-
tion can be seen from the invoice, it is not necessary to ll in this eld.
WARRANTY TERMS
Dear customers!
The warranty period is 2 years and starts from the day of
product purchase. In case of product claim, you have to show
the invoice. We kindly ask you to save the invoice!
Unfortunately, wrong handling with the device is a
reason for 95% of customer complains. You can eas-
ily avoid any problem, by getting useful information
provided by our special service department. To reach
our service department, you can call or send e-mail to
Mediblink local distributor.

16
Before sending the product back to retailer, we kindly
ask you to call our service department, to get help about
how to use the device to save you with unneeded trips.
The manufacturer guarantees free elimination of all im-
perfections due to defects in material or manufacturing
procedure by repairing or replacing the product. In case
that the product can not be repaired or replaced, the cus-
tomer will get the money refund. The guarantee is not
valid in case of the force majeure, accidents or unexpect-
ed events (such as lightning, water, re etc .), incorrect
use or incorrect transport, non-compliance with safety
and maintaining regulations or in case of unprofessional
product intervention.
Traces of every day product usage (scratches, abrasions)
and not subject to claim. The warranty does not eliminate
the customer rights, which originate from seller responsi-
bility for product aws. By accepting the claimed product
by the service department, the service department does
not take responsibility for loss of saved data or settings on
the product. All product repairs, which are performed out
of product warranty period, have to be paid by customer
by prior notice.
The manufacturer guarantees the product quality and aw-
less product operation in the warranty period, which starts
with the day of product purchase. If the product can not be
repaired in 45 days, the product will be replaced with a new
one. In case that the product can not be replaced, the mon-
ey will be refunded to the customer.
In case of product claim, call or send e-mail to Mediblink
local distributor.

17
Any serious incident that has occurred in relation to the
device should be reported to the manufacturer (Shen-
zhen Yimi Life Technology Co., Ltd.), company Mediblink
d.o.o. and the competent authority of the Member State
in which the user and/or patient is established.
Manufactured for (EU importer/uvoznik):
Mediblink d.o.o.
Gubčeva cesta 19, 8210 Trebnje, Slovenija
Mediblink®is a registered trademark.
©Packaging design is copyright. No infringements of our
trademarks and copyright material is allowed.
www.mediblink.com
Shenzhen Yimi Life Technology Co., Ltd
305, Building A, Tengbo Industrial Park
Changshangjiang Street, Longbei Village
Pingshan District
518118 Shenzhen
People’s Republic of China
Shanghai International Holding Corp. GmbH (Europe)
Eiffestrasse 80, 20537, Hamburg
Germany
Model: YM103
0123
M170

1
SLO
Mediblink pulzni oksimeter M170
NAVODILA ZA UPORABO
Prosimo, da pred uporabo izdelka v celoti
preberete navodila za uporabo
Verzija navodila za uporabo: 1.1
Datum izdaje: 17.04.2020
Datum zadnjega popravka: 04.02.2022

2
1. Predstavitev izdelka
in navodila za uporabo
1.1 Pogled od spredaj
Vrednost SpO2
Gumb
Kazalnik stanja
napolnjenosti baterije
Indeks perfuzije
Palični grafikon
srčnega utripa
Vrednost srčnega
utripa
Slika 1: Pogled od spredaj M170 (Model: YM103)
1.2Načinuporabe
A.
Odprite pokrovček predelka za baterije in vstavite
dve bateriji AAA, ki morata biti pravilno obrnjeni glede
na polarnost, ter nato ponovno namestite pokrovček.
B. Pritisnite dno naprave in razprite sondo, nato vanjo
vstavite prst.
C. Pritisnite gumb za vklop naprave in prikazal se bo
vmesnik za meritve.
D. Po približno 8 sekundah lahko z zaslona odčitate
rezultat meritve.
E. Preden odčitate parametre, se prepričajte, da so
številke na vmesniku pulznega oksimetra več kot
štiri sekunde ostale nespremenjene.
F. Ko prst umaknete s sonde, se naprava po 8 sekun-
dah samodejno izklopi.

3
1.3 Namestitev baterij
A. V prostor za baterije vstavite dve bateriji AAA, pri
čemer pazite na pravilno polarnost (slika 2).
B. Pokrovček predelka za baterije potisnite vodo-
ravno v smeri puščice, kot je prikazano na desni
strani.
OPOZORILA
• Bateriji morate vstaviti pravilno glede na pola, sicer
lahko pride do poškodb naprave.
• Če naprave dalj časa ne boste uporabljali, odstra-
nite baterije.
Slika 2: Namestitev baterij
1.4 Pritrditev traku
A. Ožji del traku vdenite skozi odprtino za obešanje.
B. Nato širši del traku speljite skozi ožji del in zateg-
nite trak (slika 3).
This manual suits for next models
1
Table of contents
Languages:
Other Mediblink Medical Equipment manuals
Popular Medical Equipment manuals by other brands

swissflex
swissflex uni 14_95RF bridge operating instructions

Villa Sistemi Medicali
Villa Sistemi Medicali STRATO 2000 Digital Service manual

Zimmer
Zimmer CryoMini operating instructions

Vive
Vive RHB1038 user manual

Respironics
Respironics REMstar Choice Service manual

VIDAR
VIDAR DiagnosticPRO Edge user guide