Medifa 63000 1 Series User manual

User manual
Version: 2.1
EN
Extension device
Article no. 63000_1, 63000_1SF


3User manual for extension device 63000_1 Version 2.1
Table of contents
Table of contents
1. Important information. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5
1.1 Revision history . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
1.2 CE label . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
1.3 Conformity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
1.4 Manufacturer and distributor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
1.5 Copyright statement . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
2. Understand the user manual . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6
2.1 List of abbreviations. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
3. Used symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7
4. Safety instructions and user obligations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8
4.1 General safety instructions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
4.2 Measures to be taken prior to each use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
4.3 Infection prevention. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
4.4 Maintenance and repair . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
4.5 Service life. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
5. Intended use. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10
5.1 Intended users . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
6. Type label . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11
7. Symbols used . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12
8. Padding . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13
9. Model description . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .14
10. Assembly. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .15
10.1 Supplied individual parts for the equipment cart . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
10.2 Mounting equipment cart . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
10.3 Symbols on the transport packaging. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16
10.4 Assembly of extension device . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17

4User manual for extension device 63000_1 Version 2.1
11. Installation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .18
11.1 Attaching the extension device to the operating table . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18
12. Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .19
12.1 General operation. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
12.2 Countertraction post for beach-chair position . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
12.3 Drawing spindle / extension shoe . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20
12.4 Adjustment options . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
13. Positioning of patient. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .22
14. Care instructions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .23
14.1 Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
14.2 Disinfection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
14.3 Drying . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
14.5 Disinfectant recommendation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 24
15. Technical data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .25
15.1 Environmental conditions for operation, storage, and transport. . . . . . . . . . . . . . . . . . . . . . . . . 25
15.2 Dimensions, weights and load limits. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
15.3 Classification . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
16. Delivery, unpacking and installation. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .26
17. Accessory list . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .27
18. Disposal. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .28
Table of contents

5User manual for extension device 63000_1 Version 2.1
Important information
Version Date Reason or modification of publication
1.0 09/2014 First edition
2.0 05/2020 Layout, content
2.1 06/2021 MDR adjustment
1. Important information
1.1 Revision history
1.2 CE label
This product is a Class 1 medical product according to the Ordinance (EU) 2017/745 on medical products and
complies with the prevailing version of this ordinance at the time of its introduction on the market.
1.3 Conformity
The manufacturer declares the conformity of this product with the essential safety and performance require-
ments according to Medical Device Regulations in Annexe I as well as the implementation of the technical
documentation according to Annexe II and declares conformity by means of an EU Declaration of Conformity
according to Annexe IV.
1.4 Manufacturer and distributor
medifa GmbH & Co. KG
Industriestraße 5
57413 Finnentrop
Germany
Telephone no.: +49 2721 7177-0
Service hotline: +49 2721 7177-410
Fax: +49 2721 7177-255
info@medifa.com
www.medifa.com
1.5 Copyright statement
This user manual including all figures is subject to copyright. The transmission and reproduction of this docu-
ment as well as the exploitation and communication of its contents are - unless expressly permitted - prohibited.
Contraventions shall impose an obligation to compensation for damages. All rights reserved in case of a patent
grant or utility model registration.
We are constantly working on the further development of our products. Please understand that we must reserve
the right to change the scope of delivery in shape, equipment, and technology at any time.
Reproduction, duplication, or translation of the original user manual, even in part, is not permitted without writ-
ten consent from medifa.
All rights according to the copyright law are expressly reserved by medifa. medifa shall only be responsible for
the safety-related properties of this device within the framework of the legal regulations if maintenance, ser-
vicing, and changes to this device are carried out by medifa itself or a representative in accordance with instruc-
tions.

6User manual for extension device 63000_1 Version 2.1
Understand the user manual
2. Understand the user manual
ATTENTION
Read and follow the instructions in the user manual
This user manual must be read and understood in full by the operating personnel before putting the extension
device into operation; this applies in particular to the chapter on safety instructions and user obligations. If
necessary, verified mediation by means of in-house training is also possible, taking into account the profes-
sional qualifications. The user manual must be strictly observed and must be available at the place of use.
ATTENTION
Using the product is safe!
Remaining residual hazards are indicated at the relevant points in the user manual.
Please observe these instructions.
2.1 List of abbreviations
Abbreviation Description
CE European Community (derived from the French "Communauté Européenne")
MDR Medical Device Regulation
7User manual for extension device 63000_1 Version 2.1
Used symbols
3. Used symbols
Various information and safety symbols are used in this user manual to highlight particularly relevant
information.
Safety Instructions
HAZARD
HAZARD indicates an imminently hazardous situation which, if not avoided, will result in death or serious
injury.
WARNING
WARNING indicates a potentially hazardous situation which, if not avoided, could result in death or serious
injury.
CAUTION
CAUTION indicates a potentially hazardous situation which, if not avoided, could result in minor or moderate
injury.
ATTENTION
Indication of harmful situation with the possible consequences: the device or something in its vicinity could be
damaged.
Instructions:
Useful instructions and further information are indicated by this symbol.

8User manual for extension device 63000_1 Version 2.1
Safety instructions and user obligations
4. Safety instructions and user obligations
4.1 General safety instructions
Keep the user manual in the vicinity of the product so that information can be read at a later date. The user man-
ual is an integral part of the product and must be handed over in case there is a change in location or personnel.
Furthermore, the user manual must be easily accessible to all users of the product at all times.
WARNING
Patient hazard!
• Any modification of the medical product is prohibited. The manufacturer shall assume no liability for chang-
es to the product.
• Hazard through incorrect handling. Please follow the instructions in the user manual of your portable oper-
ating table.
• All work with or on the product may only be carried out by trained medical or nursing staff.
• The product may only be used for the purposes indicated under Intended use. When operating the product,
the factors specified under Technical Data must be observed.
• For the intended and safe use of additional equipment, the corresponding user manual for this additional
equipment must be followed.
All mechanical functions as well as all parts of the product must be checked for functionality and integrity be-
fore each use.
The use of defective or damaged products is prohibited.
• All specifications for cleaning and disinfection must be observed.
• Observe the cleaning and disinfection procedures and agents described in this instruction manual.
• Only cleaned and disinfected devices and equipment may be handed over to a service technician or the manu-
facturer for maintenance and repair work.
• Replace padding that no longer meets the hygiene and infection prevention requirements.
Instructions to users and/or patients
All serious incidents relating to the device must be notified to the manufacturer and the competent
authority of the Member State in which the user and/or patient is located.
4.2 Measures to be taken prior to each use
4.3 Infection prevention
WARNING
Danger to the patient due to improper fastening!
• Before Each use, check if the product is securely held on the operating table.
• Check fastening elements for secure fit!
• An incorrectly secured product / accessory may come loose and cause injury. Always make sure that all
locking elements (eccentric lever, handle screws, etc.) of the product / accessory part are locked and that the
moving parts are correctly fixed. Check the locking mechanisms after each adjustment procedure.

9User manual for extension device 63000_1 Version 2.1
Safety instructions and user obligations
4.4 Maintenance and repair
To avoid failure faults and to ensure operational safety, annual maintenance must be carried out so that the
functioning is fully maintained. It is recommended to hire a medifa-certified service technician for this mainte-
nance procedure.
Repairs
Repairs may only be carried out by medifa's technical customer service department or by personnel authorised,
trained, and certified by medifa.
medifa shall not be liable for any damage caused due to deferred inspections, faulty repair or maintenance, or
changes made to the product.
For service work, please contact the medifa technical customer service department.
4.5 Service life
The product has a service life of 10 years if the specified inspection by medifa Service is observed. This excludes
all wear parts and padding.

10 User manual for extension device 63000_1 Version 2.1
The extension device is intended exclusively for human medical purposes.
The extension device may only be used with the following products:
• medifa 5000
• medifa 6000
The extension device serves, in connection with the above-mentioned products and other medifa accessories, in
the mounting, positioning and extension of the legs immediately before, during and after the execution of the
surgical intervention, as well as for examination and treatment.
The patient is positioned on the extension device according to general practice and doctrine.
The transport of patients, objects, equipment or materials on the extension device is prohibited.
The extension device may only be used / operated by trained medical and nursing staff in a responsible and
controlled manner. The training of operating and nursing personnel shall be carried out by the manufacturer or
by other persons authorised by the manufacturer.
This user manual must be followed for the intended use of the extension device.
Any other use of the product shall be deemed to be not in compliance with the intended purpose. The supplier/
manufacturer shall not be liable for personal injury or damage to property as a result of improper operation or
use.
Only accessories from the chapter Accessories list are intended for use with the extension device.
All accessories not listed may not be used for safety reasons.
Intended use
5. Intended use
5.1 Intended users
This manual describes tasks and duties for several user target groups.
We classify two user groups under healthcare professionals: Physicians, such as surgeons, orthopaedic surgeons,
and anaesthesiologists, as well as surgical assistants and nurses. The third user group consists of cleaning staff
and technicians.
All target groups must be instructed by the operator according to their field of activity, their education and pro-
fessional experience on the device in order to operate the device as intended.

11
medifa GmbH & Co. KG
Industriestraße 5 ,
57413 Finnentrop, Germany
Extension device
63000_1
xxxxx
05/2021
(01) 04250411523087
(11) xxxxx
250
User manual for extension device 63000_1 Version 2.1
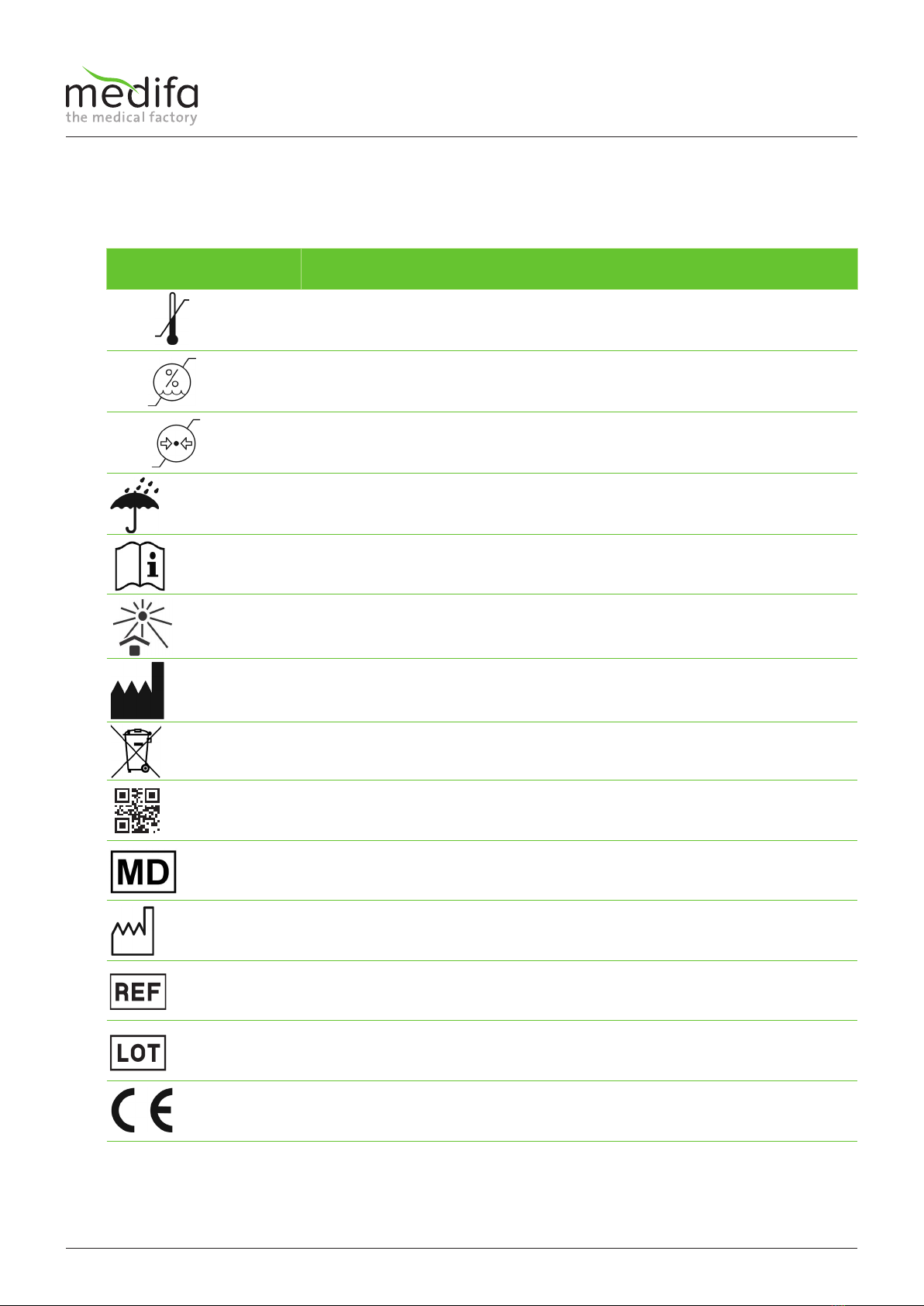
6. Type label
Type label
The type label is located under the mounting plate.
Element / symbol Description
Manufacturer details and contact information
Production period
Article number
Batch number
Data matrix code (UDI carrier)
Details of maximum additional load
Observe the safety instructions in the accompanying documents
Medical product
Read the user manual
Observe instructions for disposal
The device is declared compliant according to 2017/745 ordinance
Figure 1: Type label
12 User manual for extension device 63000_1 Version 2.1
Symbols used
7. Symbols used
On the transport packaging, there are various symbols related to handling.
Element / symbol Description
Temperature range for storage and transport -20 °C to 50 °C
Relative humidity for storage and transport 10% to 95%
Air pressure for storage and transport 70 kPa to 106 kPa
Protect transport packaging from moisture.
Read the user manual.
Protect against sunlight.
Manufacturer information
Observe instructions for disposal.
Data matrix code (UDI carrier)
Medical product
Production period
Article number
Batch number
The device is declared compliant according to 2017/745 ordinance
70 kPa
-20°C
+50°C
95%
10%
106 kPa

13User manual for extension device 63000_1 Version 2.1
Padding
ATTENTION
Do not use if the padding surface is damaged.
Operating the product with non-approved padding is prohibited and shall result in the loss of CE conformity.
The pads are made of 60 mm thick PUR padding or 80 mm thick viscoelastic foam padding. They are fastened
to the extension device by means of stud-mounting holes. The padding is disinfectable and washable as well as
radiotransparent. Furthermore, the padding is latex-free and breathable.
The padding prevents pressure necrosis of the patient. Medically trained personnel must eliminate the residual
risk through active decubitus prophylaxis when positioning the patient. The padding is antistatic and, when
fastened properly, complies with the normative regulations.
ATTENTION
Safety Instructions
• Only use the original medifa padding!
• Do not place the patient on the operating table system without padding.
• Do not insert any sharp-edged objects into the padding or place them on the padding.
• Do not attach adhesive foils.
8. Padding

14
w
e
r
t
q
y
User manual for extension device 63000_1 Version 2.1
Model description
9. Model description
Figure 2: Extension device
No. Name
1 Trolley with storage basket
2 Docking element
3 Swivelling longitudinal spar
4 Swivelling and height-adjustable drawing spindles with adaption for extension shoe holders
5 Extension shoe (accessories)
6 Supporting bars

15User manual for extension device 63000_1 Version 2.1
Assembly
10. Assembly
10.1 Supplied individual parts for the equipment cart
No. Name Quantity supplied
1 Side spars 2
2 Basket frame 1
3 Plastic guide variant A 1
4 Plastic guide variant B 1
5 Storage basket 1
6 Base with four mounted twin castors 1
7 M8 screw for basket frame 4
8 M10 screw for side spar 4
9 M8 Washer for basket frame 4
10 M10 Washer for side spar 4
10.2 Mounting equipment cart
• Screw the side spars [1] to the base using the M10
screws and washers.
• Fix the basket frame [2] to the side spars [1] using
the M8 screws and washers.
• Tighten the M10 screws of the side spars [2].
• Insert plastic guides [3 + 4] into the side spars.
• Insert equipment basket [5] into the basket frame.
w
q
e
r
t
Figure 3: Equipment cart
CAUTION
Risk of injury
Loosened locking elements can lead to injuries. Tighten all locking elements after adjustments.

16
q
w
e
r
t
y
User manual for extension device 63000_1 Version 2.1
Assembly
10.3 Symbols on the transport packaging
No. Name Quantity supplied
1 Drawing spindle 2
2 Supporting bars 2
3 Longitudinal spar 2
4 Docking element 1
5 Countertraction post 1
6 Extension shoe (optional) 2
Figure 4: Extension device (exploded view)

17
q
w
e
r
t
y
u
i
u
User manual for extension device 63000_1 Version 2.1
Assembly
10.4 Assembly of extension device
Figure 5: Structure of extension device
1. Open the locking element [1] on the left and right spar [2] of the extension device.
2. Place the left and right spar [2] on the spars of the docking element [3] and retighten both the locking ele-
ments [1].
3. Loosen the locking element [4] on the end spars [5] and place the end spars on the middle spars.
4. Open the two locking elements [6] on the end spars [5] and insert the left and right spindle unit [7] into the
circular guide. Subsequently close the respective locking elements [6] on the end spars [5] once again.
5. Then the counter traction roller [7] will be inserted into the extension device by loosening the locking element
[8] provided for this purpose and then tightened again by tightening the locking element [8].

18
qqqq
User manual for extension device 63000_1 Version 2.1
11. Installation
11.1 Attaching the extension device to the operating table
Figure 9: Installation step 4
Figure 8: Installation step 3
Figure 6: Installation step 1
Figure 7: Installation step 2
1. Push the extension device with trolley until just
before the bottom part of the operating table.
2. Adjust the height of the operating table to the
height of the square bolts of the extension
device.
3. Insert the extension device with the square bolts
into the square holes of the operating table and
push in as far as it will go.
4. Next step is to fix the star knob screws [1] on the
underside of the docking element.
5. Finally, the operating table must be raised by about 30 mm to remove the trolley.
Installation

19
ww
q
q
User manual for extension device 63000_1 Version 2.1
Operation
12. Operation
12.1 General operation
The left and right spars have a toothed joint which
is adjustable in one plane, but the spars are still ad-
justable vertically and horizontally. This is achieved
by dismantling the spar and reassembling it at the
surface rotated by 90°.
In this example in Figure 10, the right spar is adjust-
able horizontally and the left spar vertically.
To remove the spar, the rear locking element [1]
must be loosened. Accordingly, it must be fixed
during the assembly.
Adjustment in the vertical/horizontal plane
1. Hold the spar while opening the front eccentric
lever [2] to loosen the toothed joint.
2. Move the spar to the desired position.
3. Fix the eccentric lever to the toothed joint.
Figure 10: General operation
12.2 Countertraction post for beach-chair position
1. Loosen the locking element [1] to attach or re-
connect the countertraction post for the beach-
chair position.
2. Insert the countertraction post in the desired
position.
3. Tighten the locking element [1].
Figure 11: Countertraction post for beach-chair position
CAUTION
Risk of injury
Too much extension could cause injuries to the limbs. The extension device may only be operated by qualified
personnel with sufficient knowledge in this subject area.

20
e
r
w
q
User manual for extension device 63000_1 Version 2.1
Operation
12.3 Drawing spindle / extension shoe
The extension shoe is mounted on the side of the
shoe slot [1] and fixed by means of the locking
element [2]. To dismantle the drawing spindle, the
locking element [3] must be loosened.
The extension shoe can be rotated by loosening the
locking element [4], e.g. to change the system from
the normal position to the lateral position.
Figure 12: Drawing spindle
This manual suits for next models
1
Table of contents
Other Medifa Medical Equipment manuals
Popular Medical Equipment manuals by other brands

dymax
dymax BlueWave 200 user guide

Lemuria Technologies
Lemuria Technologies PEMF 4000 user manual

Otto Bock
Otto Bock 50R20 Dorso Osteo Care Instructions for use

Jumper
Jumper AngelSounds JPD-100S4 instruction manual

Allen & Handburys
Allen & Handburys Seretide Diskus Instructions for use

Joerns
Joerns Oxford Up Service manual

biodex
biodex 056-605 Installation & operation manual

3M
3M Curing Light 2500 M 5560 Instructions for use

Cardinal Health
Cardinal Health Cordis RAILWAY Instructions for use

EchoNous
EchoNous KOSMOS quick start guide
Invacare
Invacare EC-Track Installation & Technical Description

VISIOMED
VISIOMED Buddy VM-06 EVOLUTION user manual