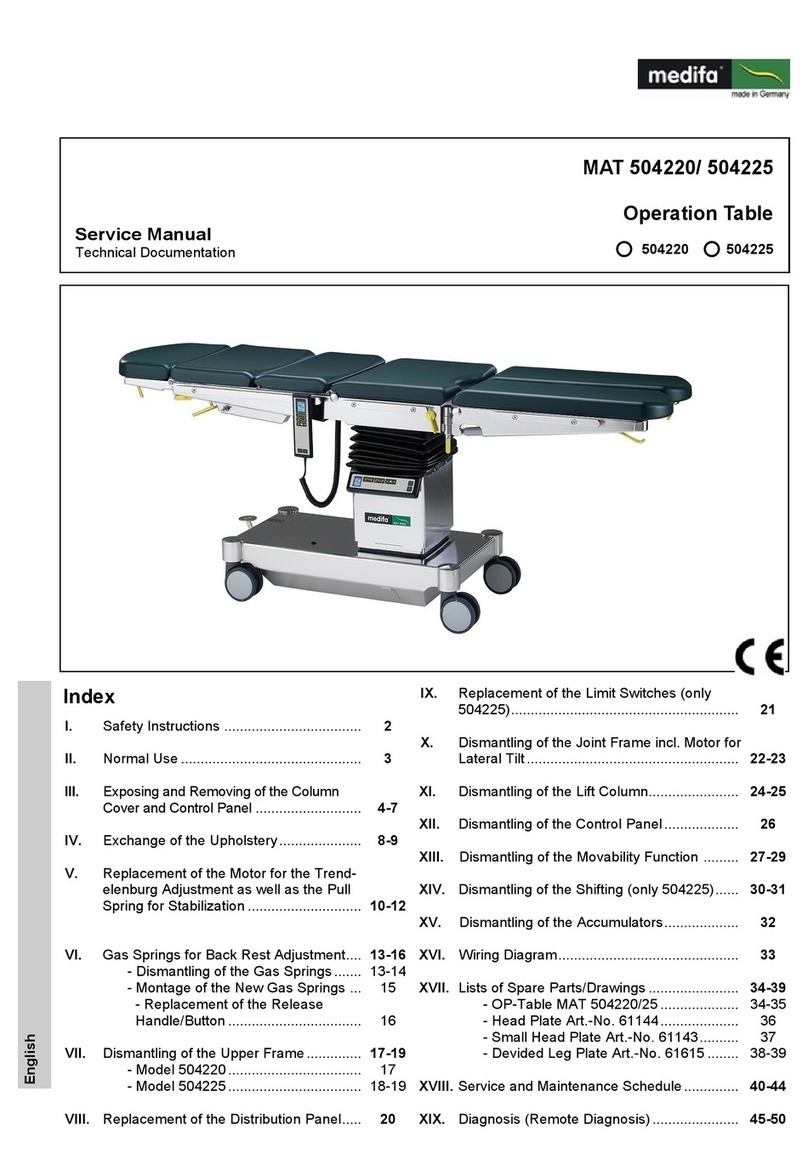
Medifa 8000 Series User manual

800200
Translation of the user manual
medifa 8000
Version: 4.0 EN


Table of contents
medifa 8000 iii
Table of contents
1 Important information.............................................................................. 7
1.1 Revision history ........................................................................................................... 7
1.2 CE marking .................................................................................................................. 7
1.3 Conformity .................................................................................................................. 7
1.4 Manufacturer and distributor ...................................................................................... 7
1.5 Copyright notice .......................................................................................................... 8
2 Foreword.................................................................................................. 9
3 Understanding these operating instructions ............................................. 10
3.1 Where can I find certain information? ......................................................................... 10
3.2 List of abbreviations .................................................................................................... 11
3.3 Symbols in use............................................................................................................. 12
4 Safety instructions and user obligations.................................................... 13
4.1 General safety instructions .......................................................................................... 13
4.2 Steps to take before each use in the OP....................................................................... 13
4.3 Risk of explosion.......................................................................................................... 14
4.4 Electricity .................................................................................................................... 15
4.5 Infection control.......................................................................................................... 15
4.6 High frequency (HF) surgery equipment and defibrillators ........................................... 15
4.7 Product lifespan .......................................................................................................... 16
4.8 Maintenance and repair .............................................................................................. 16
5 medifa 8000.............................................................................................. 17
5.1 Proper and intended usage.......................................................................................... 17
5.2 Intended users............................................................................................................. 18
5.3 medifa 8000 and components...................................................................................... 18
5.4 Warning stickers on the medifa 8000........................................................................... 20
5.5 Connections and symbols ............................................................................................ 21
5.6 Ratings plate................................................................................................................ 22

Table of contents
iv Translation of the user manual
5.7 Table top ..................................................................................................................... 24
5.8 Lifting column.............................................................................................................. 24
5.9 Base ............................................................................................................................ 26
5.10 Chassis......................................................................................................................... 27
5.11 Padded cushion ........................................................................................................... 27
5.12 Standard-compliant rails.............................................................................................. 28
5.12.1 Maximum permissible torques ........................................................................... 28
5.13 Internal and external power supplies........................................................................... 29
5.13.1 External power supply......................................................................................... 30
5.13.2 Lead-gel batteries................................................................................................ 30
5.13.3 Battery fuse ......................................................................................................... 31
5.14 Standard accessories ................................................................................................... 32
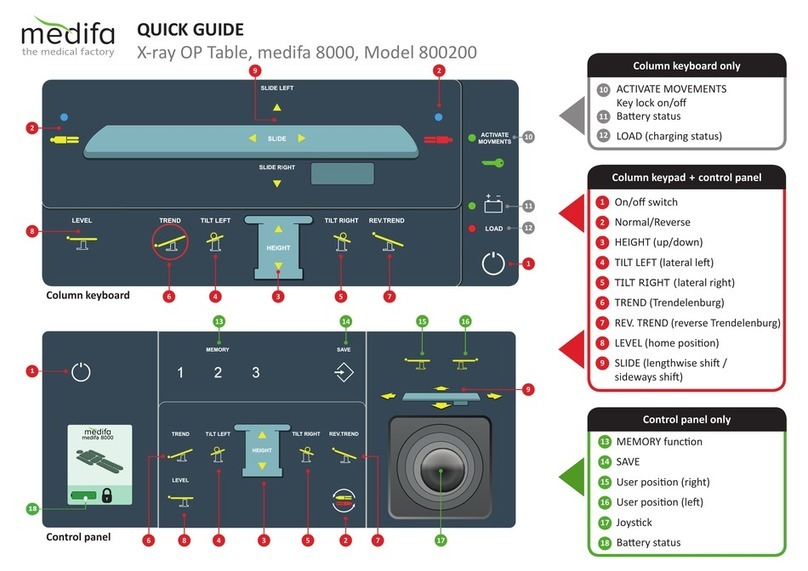
6 User controls ............................................................................................ 33
6.1 Column keypad............................................................................................................ 33
6.1.1 Operation and functionality of the keys.............................................................. 34
6.2 Control panel............................................................................................................... 37
6.2.1 Operation and functionality of the keys.............................................................. 37
6.2.2 Save the table positions (SAVE)........................................................................... 40
6.2.3 Selecting a saved table position (MEMORY) ....................................................... 41
6.2.4 Specifying the user position at the control panel ............................................... 41
6.3 Infra-red handset (optional) ........................................................................................ 42
6.3.1 Notices on the infra-red handset ........................................................................ 42
6.3.2 Charging station for the infra-red handset ......................................................... 43
6.3.3 Operation and functionality of the keys.............................................................. 44
6.4 Foot switch (optional).................................................................................................. 47
6.4.1 Operation and functionality of the pedals .......................................................... 48
7 Switching the medifa 8000 on and off....................................................... 49
7.1 Establishing the equipotential bonding........................................................................ 50
7.2 Switching on and reactivating the medifa 8000............................................................ 50
7.3 Switching off the medifa 8000 ..................................................................................... 51
8 Charging the medifa 8000 ......................................................................... 52

Table of contents
medifa 8000 v
8.1 Recognizing the battery charging mode and charging status ........................................ 54
8.2 Possible malfunctions and causes ................................................................................ 55
9 Transporting patients ............................................................................... 56
9.1 Notices on patient transport........................................................................................ 56
9.2 Transporting patients .................................................................................................. 57
10 Braking the medifa 8000 and releasing the brake ..................................... 58
11 Positioning the patient ............................................................................. 59
11.1 Preparing the medifa 8000 to hold the patient ............................................................ 60
11.2 Positioning patients..................................................................................................... 61
11.3 Normal position........................................................................................................... 61
11.4 Reverse position.......................................................................................................... 62
11.5 HEIGHT ........................................................................................................................ 62
11.6 Trendelenburg/reverse Trendelenburg (TREND/REV.TREND)....................................... 63
11.7 Lateral position (TILT RIGHT / TILT LEFT) ...................................................................... 65
11.8 Home position (LEVEL)................................................................................................. 66
11.9 Lengthwise adjustments (SLIDE) .................................................................................. 66
11.10 Sideways shift (SLIDE LEFT / SLIDE RIGHT).................................................................... 68
12 Configuring the medifa 8000..................................................................... 69
12.1 Information about the load limits ................................................................................ 69
12.2 Attaching and removing the swivelling armrest (article number 81220)....................... 69
12.2.1 Attaching the swivelling armrest......................................................................... 69
12.2.2 Adjusting the swivelling armrest......................................................................... 70
12.2.3 Removing the swivelling armrest ........................................................................ 70
12.3 Attaching and removing the turn/tilt armrest (article number 81211) ......................... 70
12.3.1 Attaching the turn/tilt armrest ........................................................................... 70
12.3.2 Adjusting the turn/tilt armrest............................................................................ 71
12.3.3 Removing the turn/tilt armrest........................................................................... 73
12.4 Attaching and removing the side extension (article number 81355)............................. 74
12.5 Attaching and removing the side support / armrest (article number 81092) ................ 75

Table of contents
vi Translation of the user manual
12.6 Attaching and removing the cushions .......................................................................... 76
12.6.1 Attaching the cushions........................................................................................ 76
12.6.2 Removing the cushions ....................................................................................... 77
13 Preparing the medifa 8000 after it has been used ..................................... 78
13.1 Cleaning and disinfecting the medifa 8000................................................................... 78
13.1.1 Cleaning............................................................................................................... 79
13.1.2 Disinfecting.......................................................................................................... 79
13.1.3 Drying .................................................................................................................. 79
13.1.4 Disposal ............................................................................................................... 79
14 Troubleshooting and service – the we care.Code ...................................... 80
15 Technical specifications ............................................................................ 82
15.1 Environmental conditions for the operation, storage and transport............................. 82
15.2 Dimensions, weights and load limits............................................................................ 82
15.3 Electrical connection data............................................................................................ 82
15.4 Adjustment ranges ...................................................................................................... 83
15.5 Classification ............................................................................................................... 83
15.6 EMC information ......................................................................................................... 84
16 Appendix .................................................................................................. 85
16.1 Delivery, unpacking and setting up .............................................................................. 85
16.1.1 Delivery ............................................................................................................... 85
16.1.2 Unpacking and setting up.................................................................................... 85
16.1.3 Symbols on the transport packaging................................................................... 86
16.2 Disposal....................................................................................................................... 87
16.3 When the device has not been used for a long time..................................................... 87
16.4 Accessories.................................................................................................................. 87

Important information
medifa 8000 7
1 Important information
1.1 Revision history
Version Date Reason for change to the publication
1.0 07/2018 First edition
2.0 11/2019 Complete revision, new cover pictures
3.0 04/2020 Main switch, mains plug
3.1 07/2020 Mains plug, pull-out protection top
4.0 08/2021 Complete revision
1.2 CE marking
This product is a Class 1 medical device as defined by European regulation (EU) 2017/745 concerning
medical devices. It complies with the version of this regulation in force at the time that this product is
placed on the market.
1.3 Conformity
The manufacturer declares that this product conforms with the essential safety and performance
requirements according to MDR Annex I. The manufacturer also declares that the technical
documentation has been implemented according to MDR Annex II. The manufacturer declares conformity
by means of an EU Declaration of Conformity according to MDR Annex IV.
1.4 Manufacturer and distributor
medifa GmbH & Co. KG
Industriestrasse 5
57413 Finnentrop
Germany
Telephone +49 2721 71770
Service hotline: +49 2721 7177 410
Fax +49 2721 7177 255
www.medifa.com

Important information
8Translation of the user manual
1.5 Copyright notice
These operating instructions and all drawings are subject to copyright. The reproduction and duplication
of this document as well as the usage and communication of its contents, are not permitted unless
explicitly stated. Violators shall be liable to pay for damages. All rights are reserved in the event that a
patent is granted or utility prototype registered.
We are constantly working on the further development and design of our products.
Please understand that we reserve the right to change the scope of delivery in terms of form,
configuration and technology at any time.
Reproduction, duplication or translation of the original operating instructions, in whole or in part, is not
permitted without the written permission of medifa!
All rights under the copyright law are expressly reserved for medifa.
medifa is only responsible for the safety characteristics within the scope of the statutory regulations if all
maintenance, servicing and changes to this device have been carried out by the user or a representative
as instructed.

Foreword
medifa 8000 9
2 Foreword
We at medifa thank you for purchasing the medifa 8000 X-ray OP table. The medifa 8000 is an X-ray OP
table that combines design, functionality and comfort with the highest quality associated with German
manufacturing.
The medifa products are manufactured to have a long and trouble-free lifespan. The development, design
and production at medifa have been certified to comply with DIN EN ISO 9001 and DIN EN ISO 13485.
These products comply with the requirements of the EU Medical Device Regulation (MDR) and bear the
CE mark.
The medifa 8000 has an ergonomically shaped table plate made of high-quality carbon that is
continuously transparent to X-rays. Its shape enables a maximum of X-ray transparency with the smallest
radiation dose. Complete 360-degree X-ray transparency is made possible by the circumferential carbon
guide rails.
The surface that the patient lies on can be shifted both vertically and horizontally. It can be controlled
using an intuitive joystick as well as the function keys on the control panel and the column keypad. You
can also take advantage of the memory feature. Up to three table positions can be stored and moved to
using a single keystroke.
The demanding requirements of the OP(surgical) sector can only be met by high-quality long-life
materials, comprehensive functionality and ease of use.
Medifa offers a complete line of accessories to meet your requirements. The periodic maintenance
carried out by our trained service technicians ensures that your X-ray OP table is always in perfect working
order during routine operations.
We are always available to help if you have any questions.

Understanding these operating instructions
10 Translation of the user manual
3 Understanding these operating instructions
ATTENTION
Please read and observe these operating instructions.
These operating instructions must be read and fully understood by the operating personnel before the
initial commissioning of the medifa 8000. This applies in particular to the 4Safety instructions and user
obligations,Page 13 chapter. If necessary, in-house training for technical qualifications may be carried
out by a qualified person.
The operating instructions must be closely followed and available at the place of use.
ATTENTION
It is safe to use the X-ray OP table!
Any remaining residual hazards are indicated at the affected locations in the operating instructions.
Follow these instructions!
3.1 Where can I find certain information?
The individual chapters of the operating instructions contain information on specific topics.
Chapter Content / topics
1 Important information ●Manufacturer's instructions
2 Foreword ●Brief description of your medifa 8000
3 Understanding these operating
instructions
●Symbols and conventions these operating instructions
4 Safety instructions and user
obligations
●Basic safety instructions for the operation and use of the
medifa 8000
5 medifa 8000 ●Description of the medifa 8000 and its components
6 User controls ●Description of the functions of the infra-red handset, column
keypad, control panel, joystick and foot switch (optional
components)
7 Switching the medifa 8000 on
and off
●Switching the power supply on/off; switching the control
unit on/off
8 Charging the medifa 8000 ●Charging the lead-gel batteries for battery-run operations
9 Transporting patients ●Moving and controlling the medifa 8000 by hand

Understanding these operating instructions
medifa 8000 11
Chapter Content / topics
10 Braking and releasing the
brakes on the medifa 8000
●Braking the medifa 8000 or releasing the brakes for driving
11 Positioning the patient ●Description of all functions for patient positioning and
position adjustment
12 Configuring the medifa 8000 ●Attaching and removing accessories and cushions
13 Preparing the medifa 8000
after it has been used
●Cleaning and maintenance after usage
14 Troubleshooting and service –
the We Care Code
●Description of the We Care Code app for troubleshooting
and service
15 Technical specifications ●Characteristics of the medifa 8000 and conditions of usage
16 Appendix ●Information on installing, decommissioning and disposing
the medifa 8000
●Accessories
Safety instructions in the chapters and steps
Notices about residual hazards and dangerous situations. Please pay particular attention to these notices.
3.2 List of abbreviations
Abbreviation Description
CE European Community (from the French "Communauté Européenne")
CDIL Continuous duty with intermittent load
DIN German Institute for Standardization
EN European Standard
EMC Electro-Magnetic Compatibility
EEC European Economic Community
HF High Frequency
IEC International Electrotechnical Commission
IP Ingress Protection
ISO International Organization for Standardization
LED Light Emitting Diode
MDR Medical Device Regulation
OP Operations (surgery)

Understanding these operating instructions
12 Translation of the user manual
3.3 Symbols in use
These operating instructions use various warning, notice and safety symbols to highlight information of
particular relevance.
Safety instructions
DANGER
DANGER indicates a hazardous situation which, if not avoided, will result in death or serious injury.
WARNING
WARNING indicates a hazardous situation which, if not avoided, could result in death or serious injury.
CAUTION
CAUTION indicates a hazardous situation which, if not avoided, could result in minor or moderate injury.
ATTENTION
Notice about a harmful situation; possible consequences: the device itself or surrounding objects could
be damaged.
Notices
Useful further information is indicated by this symbol.
Cross-references
Cross-references in the text are displayed in a different text colour and offset by a symbol: 4Positioning
the patient,Page 59
Work instructions
1. Work instructions and steps are chronologically displayed using numbered steps.

Safety instructions and user obligations
medifa 8000 13
4 Safety instructions and user obligations
Keep these operating instructions close to the product so that the information can be accessed later!
These operating instructions are an integral part of the product and must be transferred when the
location or personnel changes.
These operating instructions must also be easily accessible to all users of the product at all times.
Notice to users and/or patients
All serious incidents relating to this product must be reported to the manufacturer and to
the relevant authority of the nation in which the user and/or patient is established.
4.1 General safety instructions
WARNING
Danger for the patient!
Do not change this medical product! The manufacturer assumes no liability resulting from changes that
have been made to the X-ray OP table.
WARNING
Danger for the patient!
We cannot completely rule out the possibility of a malfunction of the medifa 8000 according to current
state of the art technology. In this rare case, the motor-driven functions would no longer be available
during the surgery.
If there is an electrical malfunction, it is possible to change the patient's position using suitable aids (e.g.
by placing cushions under the patient) or by extricating the patient from the medifa 8000.
●All work with or on the medifa 8000 (installation, commissioning, operation, maintenance,
decommissioning, transport or disposal) may only be carried out by trained medical or nursing staff.
●The medifa 8000 may only be used with the specified products for the purposes specified in the
4Proper and intended usage,Page 17 section. The values specified in 4Technical
specifications,Page 82 must be complied with whenever the medifa 8000 is being used.
●In order to use additional equipment properly and safely, the operating instructions for such
equipment must be observed.
4.2 Steps to take before each use in the OP
All electrical and mechanical functions as well as all parts of the medifa 8000 (including accessories) must
be checked for proper functionality and integrity before each use! Do not use defective or damaged
products!

Safety instructions and user obligations
14 Translation of the user manual
4.3 Risk of explosion
There is a risk of explosion if the medifa 8000 is used under one or more of the following conditions: The
following operating situations must absolutely be avoided within the AP-M zone:
●Operating under mains or battery power while in an oxygen-enriched room.
●Using the mains power supply in rooms that are enriched with anaesthetics.
●If operating from the mains power supply: The user or other person should unplug the power cord
when using the table in a room with oxygen-enriched air.
Definition of a hazardous explosive area (AP-M zone)
The AP-M zone is the medical zone shown in red in the following figure.
Figure1: Hazardous explosive area, AP-M zone
Technical specifications and further information on explosion protection can be found under
4Classification,Page 83.

Safety instructions and user obligations
medifa 8000 15
4.4 Electricity
The electrical safety of the medifa 8000 and your power supply should be checked before first usage and
regularly thereafter by a qualified electrician. We recommend that a general safety inspection be carried
out yearly by medifa's technical support.
●Do not crush or run over mains power cables or equipotential bonding cables. Do not use cables which
have been damaged.
●Remove the cables before changing the location of the table.
●Electrical discharges to the patient or operator may occur if there is no earthing/grounding present.
If you have any doubts about the safety of the mains power cables or equipotential bonding cables,
Use only the internal power supply until the problematic cable has been replaced.
●Only use the medifa 8000 on an electrically conductive floor and with an equipotential bonding cable
connected.
4.5 Infection control
●Follow all regulations for cleaning and disinfection!
●Follow the cleaning and disinfection procedures described in these operating instructions! Use only
the specified cleaning agents!
●Only cleaned and disinfected devices and equipment may be transferred to a service technician or the
manufacturer for maintenance and repair work!
●Replace any cushioning that no longer complies with the relevant hygiene and infection requirements!
4.6 High frequency (HF) surgery equipment and defibrillators
The medifa 8000 may be used with HF surgical devices, defibrillators and defibrillator monitors. Observe
the operating instructions and safety instructions from the manufacturers of these devices!
DANGER
Danger of patients being burned
The use of HF surgery equipment, defibrillators, and defibrillator monitors may pose a risk of the patient
being burned if no safety precautions are taken. Take the following security precautions:
–Position the patient on the medifa 8000 so that they are isolated from (not touching) the metal parts
(medifa 8000 or accessories), conductive cushions or hoses.
–Do not touch the patient with moistened wipes or other moist materials. Use only dry materials!
WARNING
Danger for the patient!
The electro-motor functions of the medifa 8000 may be interrupted while high-frequency surgery
devices are being used.

Safety instructions and user obligations
16 Translation of the user manual
4.7 Product lifespan
The medifa 8000 has a lifespan of 10 years if the specified inspections are carried out by medifa service
technicians.
This does not apply to all wearing parts such as drives, controls, padded cushions or keys on the control
units. It also does not apply to separate components such as the infra-red handset, control panel, foot
switch or joystick.
4.8 Maintenance and repair
To avoid malfunctions and to ensure operational safety (e.g. for lengthwise adjustments), annual
maintenance (electrical wiring, worn bearings, etc.) must be carried out to ensure that all functionality is
fully retained. We recommend that a medifa-certified service technician carry out this service work.
Repairs
Repairs may only be carried out by the medifa technical support or by personnel who have been
authorized, trained and certified by medifa.
The company medifa is not liable for any damages due to neglected inspections, inadequate repair,
improper maintenance or changes made to the product!
For servicing work, please contact the medifa technical support department.
Telephone number of the medifa service hotline: +49 2721 7177 410

medifa 8000
medifa 8000 17
5 medifa 8000
5.1 Proper and intended usage
The products described here are intended only for human medical purposes!
The 800200 X-ray OP table, together with the other accessories from medifa, is intended only for the
following uses:
●For supporting patients from the initiation of anaesthesia, during the operation, and until the end of
anaesthesia.
●For transporting patients on the X-ray OP table from a transfer facility to the operating room or from
the operating room to the transfer facility (depending on the patient transport conditions).
●For supporting patients during X-rays.
The 800200 X-ray OP table is intended for the following indications:
●Cardiovascular surgery
●Vascular surgery
●Neuro-/spinal column surgery
No contra-indication is known.
Patients should be supported and positioned on the X-ray OP table according to general practice and
training standards. The X-ray OP table must be covered with sterile material before use.
Transporting objects, equipment or materials on the X-ray OP table is prohibited. The X-ray OP table may
only be used and operated responsibly and in a controlled manner by trained medical and nursing staff.
The manufacturer of other persons authorized by the manufacturer shall carry out the training for the
operating and care personnel.
These operating instructions must be followed in order to ensure proper and intended usage of the X-ray
OP table! Any other usage of the X-ray OP table is considered improper! The supplier/manufacturer is not
liable for personal injury or property damage as a result of improper operations or usage.
Only those accessories listed in the appendix should be used with the X-ray OP table. For safety reasons,
you should not use unlisted accessories.

medifa 8000
18 Translation of the user manual
5.2 Intended users
These operating instructions describe the tasks and responsibilities relevant to multiple user groups.
We have identified two groups of users among the healthcare professionals. Doctors, such as surgeons,
orthopaedics and anaesthetists, as well as operating assistants and nursing staff. The third group of users
consists of the cleaning staff and technicians.
All target groups shall be instructed by the operating company according to their field of activity, their
training, and their professional experience on the device so that they can operate the device properly and
as intended. Technicians may only have secondary information because there are separate instructions
that specifically targets this audience.
5.3 medifa 8000 and components
The medifa 8000 X-ray OP table consists of a continuous carbon table plate. The control panel with
joystick is located on the table top. An infra-red handset and a foot switch can also be configured to
control the medifa 8000. The column keypad is built into the lifting column.
Model 800200
Figure2: medifa 8000 (Model 800200)
qOne-piece carbon table plate with built-in
standard rails
wAdjustable panels
eControl panel with joystick rLifting column
tPedal for the central brake yBase
uColumn keypad

medifa 8000
medifa 8000 19
Features
●Control panel with colour display and built-in joystick
●Electrically conductive cushions (available in different thickness)
●Full carbon plate with built-in carbon standard guide rails for accessories
●Batteries and charger are built into table (with a capacity for approx. 1 week in normal operating
mode).
●The control unit for electrical functions is on the lifting column (column keypad)
●Interface ports for the infra-red handset and foot switch
●Basic configuration: Visco-elastic comfort cushions with memory effect: 20 mm height, electrically
conductive
●Option for reverse positioning of patient
●Height adjustable from 750 to 1110 mm
●Trendelenburg adjustment of max. +/- 25°
●Lateral adjustment of max. +/- 20°
●Automatic, simultaneous horizontal (home) position for the lying surface
●Maximum permissible total load: 250 kg
●Own weight: 250 kg
●Lying surface (L x W): 2335 x 500 mm
●Continuously radiolucent carbon table plate
●Front guide rail (EU/ EU) made only from carbon; for attaching the head-positioning segments
●Lengthwise adjustment of the table top: 300 mm
●Sideways adjustment of the table top: 180 mm
●Save and retrieve any three application functions (memory feature)
●Can be driven/moved, with central brake
●Mains power: 100 - 240 V / 50 - 60 Hz
●Battery capacity displays on control panel and column keypad

medifa 8000
20 Translation of the user manual
5.4 Warning stickers on the medifa 8000
Observe the warning symbols (stickers) on the medifa 8000; they indicate the danger points and hazards.
All warning symbols on the product must be complete and legible. They must not be altered, removed or
covered by other objects. Damaged or detached warning symbols must be replaced according to the
specifications.
Figure3: Warning stickers on the medifa 8000
Symbol Description
Patients may not climb onto or sit in this area of the table top.
There is a risk of injury if the medifa 8000 is tilted
The medifa 8000 may only be used on surfaces that have no more than a 5 degree tilt.
Read the instructions before use.
Other manuals for 8000 Series
1
This manual suits for next models
1
Table of contents
Other Medifa Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Harvest Healthcare
Harvest Healthcare BLENHEIM ACTIVE SEAT CUSHION General User/ Safety Guide

3Disc
3Disc FireCR Flash user manual

bort medical
bort medical 930 140 quick guide

EMC Security
EMC Security belle user guide
DJO
DJO ARTROMOT-S3 operating instructions

Covidien
Covidien Nellcor D-YSPD Instructions for use