MedRx Avant A2D+ User manual


◦ ◦
◦◦

D-0117966-B 01/2019 3

4 D-0117966-B 01/2019
•

D-0117966-B 01/2019 5

6 D-0117966-B 01/2019
Unplug both the Red & Blue connectors. (see above)
Place a small flat head screw driver on the small orange
tabs and push down while inserting a speaker wire into
the opening then remove the screw driver. Be sure the
wire is secure.
Repeat until all the speaker wires are secured, then plug
both connectors into the device as indicated above.
Screw
Driver
Screw
Driver

D-0117966-B 01/2019 7
Use the accessories provided with your Audiometer. Use of unapproved accessories is not recommended.
* HDA 300 earphones must be
ordered with the Stealth High
Frequency Option Upgrade.

8 D-0117966-B 01/2019
My Computer
Setup
Install
AVANT Audiometer.
Manual.
Next.

D-0117966-B 01/2019 9
I accept …
Next.
Next.
Next.
Change

10 D-0117966-B 01/2019
Install.
Back.
Finish.

D-0117966-B 01/2019 11
Close.
AVANT Audiometer
Advanced Options

12 D-0117966-B 01/2019
Audio tab.
4. Audio Properties.
5.
Set Default.
OK.

D-0117966-B 01/2019 13
Function.
Calibrate.
Load Calibration.
Load.
YES

14 D-0117966-B 01/2019
Guidance and manufacturer’s declaration – electromagnetic emissions
The Avant Audiometer is intended for use in electromagnetic environment specific below. The customer or the user of
the Avant Audiometer should assure that it is used in such an environment.
Emission test
Compliance
Electromagnetic environment - guidance
RF emissions
CISPR 11
Group 1
The Avant Audiometer uses RF energy only for
its internal function. Therefore, its RF emissions
are very low and
are not likely to cause any interference in nearby
electronic equipment.
RF emissions
CISPR 11
Class B
The Avant Audiometer is suitable for use in all
establishments, including domestic
establishments and those directly connected to
the public low - voltage power supply network
that supplies buildings used for domestic
purposes.
Harmonic emissions
IEC 61000-3-2
Non applicable
Voltage fluctuations / flicker emissions
IEC 61000-3-3
Non applicable
Guidance and manufacturer’s declaration – electromagnetic immunity
The Avant Audiometer is intended for use in electromagnetic environment specific below. The customer or the user of
the Avant Audiometer should assure that it is used in such an environment.
Immunity test
IEC 60601
test level
Compliance level
Electromagnetic
environment - guidance
Electrostatic discharge
(ESD)
IEC 61000-4-2
+/- 6 kV contact
+/- 8 kV air
+/- 6 kV contact
+/- 8 kV air
Floors should be wood,
concrete or ceramic tile. If
floors are covered with
synthetic material, the
relative humidity should be
at least 30%.
Electrical fast transient /
burst
IEC 61000-4-4
+/- 2 kV for power supply
lines
+/- 1 kV for input / output
lines
+/- 2 kV for power supply
lines
+/- 1 kV for input / output
lines
Mains power quality should
be that of a typical
commercial or hospital
environment.
Power frequency (50/60 Hz)
Magnetic field
IEC 61000-4-8
3 A/m
3 A/m
Power frequency magnetic
fields should be at levels
characteristic of a typical
location in a typical
commercial or hospital
environment.
Guidance and manufacturer’s declaration –electromagnetic immunity
The Avant Audiometer is intended for use in electromagnetic environment specific below. The customer or the user of
the Avant Audiometer should assure that it is used in such an environment.
Immunity test
IEC 60601- test level
Compliance level
Electromagnetic environment - guidance
Portable and mobile RF communications
equipment should be used no closer to any
part of the Avant Audiometer, including
cables, than the recommended separation
distance calculated from the equation
applicable to the frequency of the transmitter.
Recommended separation distance:
Conducted RF
IEC 61000-4-6
3 Veff
3 Veff
d = 1,17
P

D-0117966-B 01/2019 15
Radiated RF
IEC 61000-4-3
3 V/m
80 MHz to 2,5 GHz
3 V/m
d = 1,17
P
80 to 800 MHz
d = 2,33
P
800 MHz to 2,5 GHz
Where P is the maximum output power rating
of the transmitter in watts (W) according to the
transmitter manufacturer and d is the
recommended separation distance in meters
(m).
Field strengths from fixed RF transmitters, as
determined by an electromagnetic site survey
a, should be less than the compliance level in
each frequency range b.
Interference may occur in the vicinity of
equipment marked with the following symbol:
NOTE 1 At 80 MHz and 800 MHz, the higher frequency ranges applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and
reflection from structures, objects and people.
a Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land
mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically
with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site
survey should be considered. If the measured field strength in the location in which the Avant Audiometer is used
exceeds the applicable RF compliance level above, the Avant Audiometer should be observed to verify normal
operation. If abnormal performance is observed, additional measures may be necessary, such as reorienting or
relocating the Avant Audiometer.
b Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
Recommended separation distances between
Portable and mobile RF communications equipment and the Avant Audiometer
The Avant Audiometer is intended to use in an electromagnetic environment in which radiated RF disturbances are
controlled. The customer or the user of the Avant Audiometer can help prevent electromagnetic interference by
maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the
Avant Audiometer as recommended below, according to the maximum output power of the communications
equipment.
Rated
maximum
output power
of transmitter
W
Separation distance according to frequency of transmitter
meters
150 kHz to 80 MHz
d = 1,17
P
80 MHz to 800 MHz
d = 1,17
P
800 MHz to 2,5 GHz
d = 2,33
P
0,01
0,12
0,12
0,233
0,1
0,37
0,37
0,74
1
1,17
1,17
2,33
10
3,7
3,7
7,40
100
11,7
11,7
23,3
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in meters
(m) can be estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output
power rating of the transmitter in watts (W) according to the transmitter manufacturer.
NOTE 1 At 80 MHz and 800 MHz the separation distance for the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption
and reflection from structures, objects and people.

16 D-0117966-B 01/2019
Regarding electrical safety, this device is designed to be used only by professionals in the hearing
healthcare industry.
It is Class II Medical Electrical (ME) equipment that is part of an ME system. This device provides
Type B protection (Type B equipment, Type B applied part)
This device is not protected from ingress of water.
Power is supplied by the USB cable connected to a computer.
A USB Optical Isolator, with a minimum of 1500V AC isolation and should be placed in-line between the
computer’s USB connection and the MedRx device. The Optical Isolator should be powered by a power
supply that conforms to IEC 60601-1. The computer, Optical Isolator’s power supply and the speaker’s
power supply should be connected to the Medical Grade isolation transformer that conforms to IEC 60601-1.
Follow the manufacturer’s instructions for installation and use. All connected equipment provides 2 MOPP
per IEC 60601-1.
This device is to be operated on non-conductive surfaces only.
The computer used with this device should conform to the requirements of IEC 60601-1.
A MULTIPLE PORTABLE SOCKET-OUTLET or extension cord shall not be connected to the system.
The device warm-up time is less than 2 minutes.
Do not connect items that are not specified as part of the system.
The use environment should be between 10°C and 35°C , humidity within 30% to 90%
and an atmospheric pressure range from 80 kPa to 104 kPa.
Storage temperature range at least from -20°C to 50°C and humidity level from 10% to 90%.
All components with patient contact are made of bio-compatible materials.
This device does not produce any adverse physiological effects.
Install the device as directed by this manual to achieve optimal use. Clean accessories per the cleaning
instructions prior to use. No Sterilization is required for components of this device. However, new foam
inserts are needed for each patient where applicable and cleaning of the device and accessories should
follow the procedure outlined below.
The device is not intended to be operated in an environment with anesthetics, oxygen or NO. It is not an AP
or APG device. This ME System is not intended for use with flammable anesthetics.
This device uses Type B application parts temporarily placed on the patient during testing. They are
nonconductive and can be immediately withdrawn from the patient at any time.
The device is intended for continuous operation.
The computer and the MedRx device or accessories may be located in the patient environment if required.
The colored lights are as designated by ANSI S 3.6 and IEC 60645-1. They signify that either the left (blue)
channel is active or the right (red) channel is active, or no channel is active (green). The colors do not signify
any dangerous or faulty condition.
Contact the local MedRx distributor for safe and proper disposal of this equipment. Proper disposal
may require that it be sent to collection facilities for recovery and recycling.
All repairs should be sent to MedRx for evaluation and / or repair. However, necessary diagrams and repair
instruction will upon request be provided to authorized repair personnel.
There are no known contraindications for the use of this equipment.
The Instructions for Use (the Installation and Software Training manuals) are supplied as an electronic copy
on a USB flash drive. Paper copies of the manuals may be also requested from the company, and will be
sent within one business day of the request.
D-0117966-B 01/2019 17
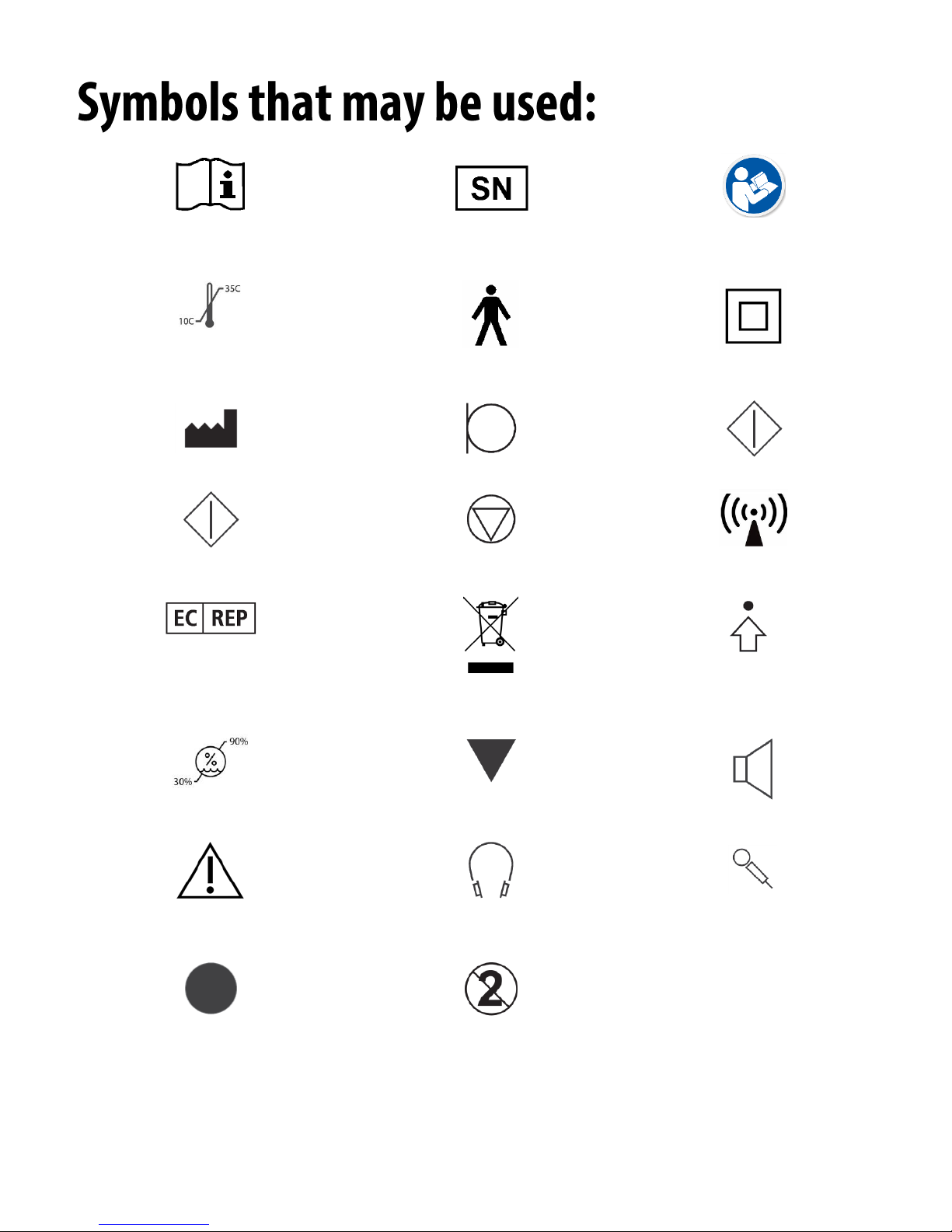
Read the instruction manuals
for safe usage of the device
(operating instructions)
or SN Indicates that the device
serial number will follow.
Read the instruction manuals
for safe usage of the device
(operating instructions)
Temperature limitation
Type B applied part.
(Type B equipment)
Class II equipment
Manufacturer (MedRx)
Microphone
Start (of action)
Start (of action)
Stop (of action)
Non-ionizing
electromagnetic radiation
Authorized Representative
in Europe
Special Disposal Required
Percentile Setup
Humidity Limitation
Calibration
Loudspeaker (Speaker)
Caution, General warning sign
Headphones
Handheld microphone
(Talkback Microphone)
Recording
CAUTION
For Single Patient Use Only

18 D-0117966-B 01/2019

D-0117966-B 01/2019 19
Other manuals for Avant A2D+
5
This manual suits for next models
1
Table of contents
Other MedRx Measuring Instrument manuals

MedRx
MedRx Avant Stealth User manual

MedRx
MedRx AVANT REM Speech+ Installation guide

MedRx
MedRx Avant Installation guide

MedRx
MedRx AVANT ARC Installation guide

MedRx
MedRx AVANT Series User manual

MedRx
MedRx Avant A2D+ Installation guide

MedRx
MedRx AVANT Stealth A2D User manual

MedRx
MedRx Avant User manual